- Home
- Industry
- Applications and case studies
- Pharmaceuticals and Biotech
Pharmaceuticals and Biotech
Pharma and biotech firms make wide use of synchrotron X-rays for drug discovery, drug development and drug delivery systems, as well as health care technologies such as implants and radiotherapy.
For drug discovery, the ESRF offers state-of-the-art protein crystallography facilities, including MASSIF, the world-leading unique beamline for the high throughput, fully automatic characterisation and data collection of macromolecular crystals. The ESRF produces more than half of the protein structures deposited in Europe. Thousands of samples are screened every day with high-throughput screening techniques.
Thanks to the automation of our facilities, protein crystallography measurements are possible through both mail-in and remote access from your home lab.
The other techniques most used by pharma and biotech companies are small/wide and ultra-small angle X-ray scattering, pair distribution function (PDF), micro- and nano-3D tomography and X-ray fluorescence and powder diffraction.
Characterisation of pharmaceutical active ingredients and formulas in situ and their behaviour in different conditions.
Studying drug delivery vectors such as nano-holders and phospholipid/micellular structures.
Resolving the crystal structures of drug formulations.
CASE STUDIES
Company or institute
Sanofi Pasteur Ltd
Challenge
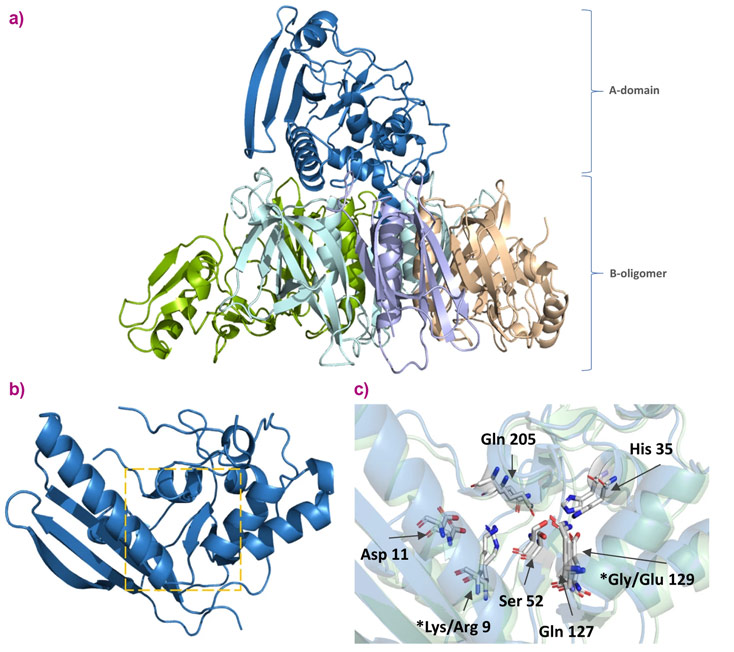
Whooping cough is caused by the bacterium Bordetella pertussis. It is a disease that affects 16 million people worldwide every year, mostly children under 10 years, resulting in about 60,000 deaths. Antibiotics are generally ineffective against the infection. Prevention of the disease through vaccination is successful, although current vaccines are not particularly efficient and require multiple doses to stimulate a good immune response.
Pertussis toxin (PTx), an exotoxin, is an important virulence factor of B. pertussis. A genetically detoxified variant of the toxin (gdPT) is an attractive candidate for the development of improved pertussis vaccines as it could retain more structural properties of native PTx than the chemically denatured native toxin currently (PTd) used in vaccines. The challenge at the ESRF was to obtain the crystal structure of gdPT so that researchers could compare it with the known wild type pertussis toxin structure. It had been hypothesised that the active site of gdPT has been disrupted by the two mutations present. A comparison of the crystal structures aimed to clarify this.
Sample
Purified PTx, PTd, and gdPT were produced by Sanofi Pasteur Ltd. Crystals of the proteins were obtained using standard methods.
Solution
Fully automatic data collection was carried out at beamline MASSIF-1 (ID30A-1), and the structures solved using molecular replacement. The structure of gdPT was obtained at a resolution of 2.13 Å.
Benefits
The crystal structure of gdPT was found to be nearly identical to that of pertussis toxin. The two mutation sites are located outside of the active site pocket. Their effect is believed to change the surrounding hydrogen-bonded network, potentially affecting cofactor binding and substrate access. Additionally, various biochemical assays were used to demonstrate that gdPT can still generate an immune response. This research could lead to improved vaccines against whooping cough.
Reference
Genetically detoxified pertussis toxin displays near identical structure to its wild-type and exhibits robust immunogenicity, S.F. Ausar, S. Zhu, J. Duprez, M. Cohen, T. Bertrand, V. Steier, D.J. Wilson, S. Li, A. Sheung, R.H. Brookes, A. Pedyczak, A. Rak & D.A. James, Commun Biol 3, 427 (2020). DOI: 10.1038/s42003-020-01153-3.
Company or institute
Carbios and the Toulouse Biotechnology Institute, Université de Toulouse
Challenge
A means to recycle plastics in an environmentally friendly way has been the holy grail of biotechnology. Thermomechanical recycling of poly(ethyleneterephthalate)(PET) bottles produces a material of insufficient quality for the synthesis of new bottles and therefore the majority of used bottles ends up in landfill disposal. Carbios, in collaboration with the Toulouse Biotechnology Institute, set out to engineer an efficient PET depolymerase starting from an enzyme with a known structure and depolymerase activity, however, insufficient for industrial use. The researchers engineered a new enzyme for higher thermal stability and higher activity permitting a 100-fold productivity improvement compared to previously published results. A crystal structure of the new PET depolymerase was needed for confirmation of the modifications to the structure through bioengineering.
Sample
The engineered PET depolymerase was expressed in E. Coli. Following purification, it was crystallised using standard methods.
Solution
Data collection was carried out at beamline ID30B and the crystal structure obtained through molecular replacement starting from the known structure of the wild-type enzyme.
Benefits
The crystal structures were taken as input for molecular dynamics simulations, which together provided insight into the structural effect of the mutations generated through bioengineering of the enzyme.
This research was concluded by a successful pilot-scale depolymerisation of PET waste reaching 90% conversion to terephthalic acid in 10 hours, which, following purification (99.8% pure), was demonstrated as suitable raw material for the fabrication of new PET bottles.
Reference
An engineered PET depolymerase to break down and recycle plastic bottles, V. Tournier, C.M. Topham, A. Gilles, B. David, C. Folgoas, E. Moya-Leclair, E. Kamionka, M.-L. Desrousseaux, H. Texier, S. Gavalda, M. Cot, E. Guémard, M. Dalibey, J. Nomme, G. Cioci, S. Barbe, M. Chateau, I. André, S. Duquesne & A. Marty, Nature 580, 216–219 (2020); doi: 10.1038/s41586-020-2149-4.
Company or institute
Sanofi, France in collaboration with the Institute for Research in Biomedicine, Università della Svizzera Italiana, Switzerland.
Challenge
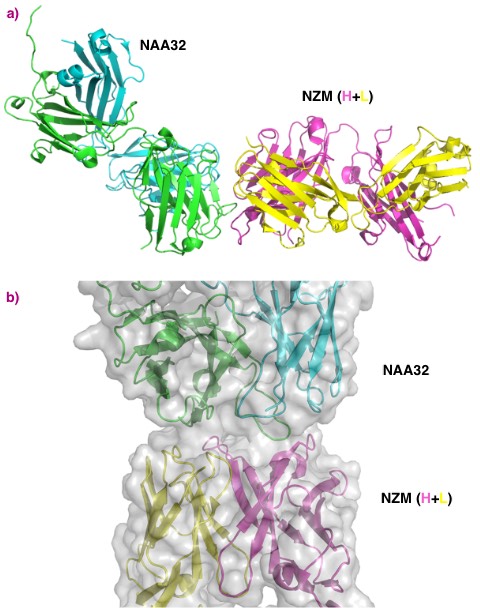
Natalizumab is a therapeutic antibody used to treat multiple sclerosis. The treatment has to be discontinued in about 6% of the patients because their immune system produces antibodies against natalizumab that reduce its efficacy. Researchers studying the interaction needed structural data on the natalizumab/antibody complex to complement peptidomics and in-silico predictions.
Sample
Stable complexes of natalizumab, itself a humanised monoclonal antibody, and patient derived anti-natalizumab antibodies were isolated and crystallised.
Solution
Beamline MASSIF-1 (ID30A-1) was used for fully-automated data collection on individual crystals.
Benefits
Crystal structures were obtained to 2.8 Å resolution or better. This permitted the interaction of natalizumab and the patient-derived antibodies to be determined and the structural features of the binding region identified. Both anti-natalizumab antibodies were found to bind to the same surface area on natalizumab but with specific differences that helped to explain different binding affinity and differences between the patients response to treatment.
Reference
A single T cell epitope drives the neutralizing anti-drug antibody response to natalizumab in multiple sclerosis patients, A. Cassotta, V. Mikol, T. Bertrand, S. Pouzieux, J. Le Parc, P. Ferrari, J. Dumas, M. Auer, F. Deisenhammer, M. Gastaldi, D. Franciotta, C. Silacci-Fregni, B. Fernandez Rodriguez, I. Giacchetto-Sasselli, M. Foglierini, D. Jarrossay, R. Geiger, F. Sallusto, A. Lanzavecchia and L. Piccoli, Nature Medecine 25, 1402-1407 (2019); doi: 10.1038/s41591-019-0568-2.
Company Genentech, Inc. Challenge Chronic pain is an important and unmet medical need that affects the quality of life for millions of people worldwide. Available treatment options include opioids and non-steroidal anti-inflammatory drugs that can be effective but often have unwanted side effects, limiting their therapeutic utility. Genetic studies in humans have recently identified a mutation in the voltage-gated sodium (Nav) channel, Nav1.7, which causes people to lose the ability to feel pain. Intense efforts have been underway to identify antagonists that selectively inhibit only the Nav1.7 channel but leave the other eight human Nav channel isoforms unaffected. It has been a major challenge to identify selective Nav1.7 channel inhibitors because all nine Nav channel isoforms are highly related in sequence, and all clinically available Nav channel blockers are poorly selective. Sample Nav1.7 contains four peripheral voltage-sensor domains (VSDs) that surround and control a central ion pore domain that allows sodium ions to enter and initiate action potentials in sensory neurons. Researchers from Genentech, in collaboration with Xenon Pharmaceuticals, wanted to study a new class of inhibitors that appear to target the fourth voltage-sensor domain (VSD4) and selectively inhibit the Nav1.7 channel. Because high-resolution structural studies of the human Nav1.7 channel are hindered by the molecules complexity, these researchers exploited a simpler bacterial Nav channel by fusing portions of human Nav1.7 (i.e. VSD4) onto it. Solution The Nav1.7 VSD4-bacterial channel fusion protein was crystallized and high-resolution diffraction studies were conducted at beamline ID29. The resulting crystal structures revealed the details of how the new class of inhibitor directly interacts with residues within the fourth voltage-sensor domain to selectively and potently inhibit the Nav1.7 channel. Benefits The new structures provide insight into the mechanism of voltage sensing and can enable the design of more selective Nav channel antagonists. Hopefully these results may accelerate the development of treatments for pain that selectively target Nav1.7 and aid drug design efforts aimed at other voltage-gated ion channels.
Science, Vol. 350, Issue 6267, DOI: 10.1126/science.aac5464. |
Companies
Technologie SERVIER and Novitom
Challenge
Curing is used to stabilise coatings to enhance tablet long-term stability and to ensure reproducible dissolution time. Technologie SERVIER wanted to investigate the effect of curing on the structure of a cellulose-based tablet coating, and in particular to determine the optimal duration for dynamic curing.
Sample
Tablets containing a freely-soluble drug were provided by SERVIER. The materials used to coat the tablets were commercially available. The coated tablets were dynamically cured for durations between 0 to 6 hours.
Solution
X-ray micro-computed tomography (XµCT) at beamline ID19 was used to examine the coating of the tablets. This non-destructive method revealed information about the internal porosity of the coating and provided observation of the qualitative differences between tablets with various degrees of curing. Regions of crystalline and amorphous matter were clearly visible along with the pore structure.
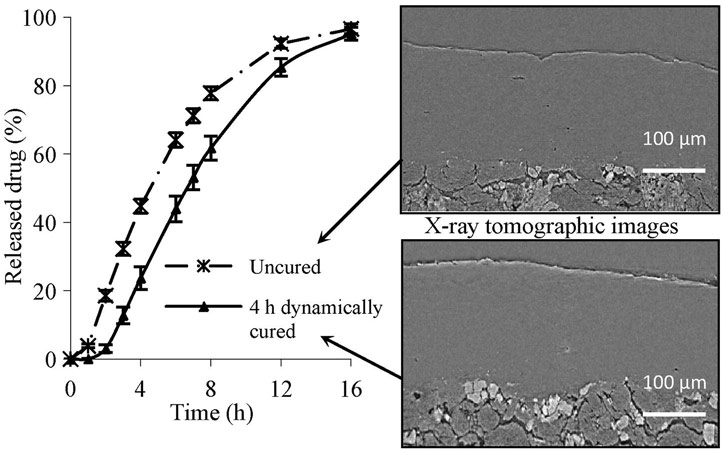 |
|
Legend. Reconstructed XµCT cross-sectional images obtained from uncured and cured coated tablets and presented with drug dissolution profiles. The XµCT data were collected with a voxel size of 0.28 µm. Reprinted with permission from Elsevier. |
Benefits
Using XµCT, the decrease in internal porosity was observed with increased curing time. The porosity volume provided a more precise determination of the optimal curing time than traditional dissolution testing alone.
Reference
Comprehensive study of dynamic curing effect on tablet coating structure, C. Gendre, M. Genty, J. César da Silva, A. Tfayli, M. Boiret, O. Lecoq, M. Baron, P. Chaminade, J.M. Péan, European Journal of Pharmaceutics and Biopharmaceutics 81, 657–665 (2012); doi: 10.1016/j.ejpb.2012.04.006.
Company
CRELUX GmbH (a WuXi AppTec Company)
Challenge
AMPK (AMP-activated protein kinase) is a Ser/Thr kinase composed of two regulatory subunits and a catalytic subunit that together as a complex regulates the levels of energy in the cells. This complex is evolutionarily conserved and ubiquitously expressed. There are a total of 12 possible isoforms, which are distributed in the human body in a tissue-specific manner. For example, one isoform is highly expressed in skeletal muscles and a different isoform is more specific to heart, brain or liver. All in all, AMPK senses the energy levels of the cells (in the form of the so-called ATP) and allows upstream signals to activate it, in response to external nutritional stress. AMPK substrates are involved in lipid metabolism, autophagy, mitochondrial biogenesis, and in the maintenance of glucose homeostasis. Therefore, this complex is a highly promising therapeutic drug target against diabetes, obesity, Wolff-Parkinson-White Syndrome, cancer, and aging.
There are, however, several challenges to generate soluble and stable complexes, and this is one of the many reasons why not many X-ray structures are available, especially at resolutions suitable to drive drug discovery efforts. Firstly, it is very complicated to be able to make crystals of the complex with the activated compound bound to it. Another obstacle is the extreme sensitivity of the tiny crystals to radiation damage.
CRELUX/ WuXi AppTec, a company expert worldwide in premium drug discovery solutions for global pharma, biotech and research organizations, came to the ESRF to tackle this challenge.
Sample
CRELUX/WuXi AppTec used their expertise to design and produce a fully functional AMPK complex with the needed yield, purity, and specific post-translational modifications for successful crystallization and X-ray structure determination of an active complex. The AMPK sample contains an kinase activating compound and three AMP nucleotides (ATP-depleted scenario) bound to it.
Solution
CRELUX/WuXi AppTec scientists sent crystallized AMPK samples to the ESRF’s ID30-A beamline. Because of the complexity of the project, they needed a powerful beamline, state-of- the-art detector and a skillful scientist in-house to carry out the experiment. They managed to solve the structure of the complex at a resolution of 2.9 Angstroms, which was enough for CRELUX/WuXi AppTec to see the detailed chemical enviroment of the compound in the complex binding site. This corresponds to one of the highest resolution structure published so far for any AMPK isoform.
Benefits
The work at the ESRF will help CRELUX/WuXi AppTec to support their clients in the discovery and development of novel and more specific drugs that can influence AMPK activity in the cell and, as a result, adjust the energy balance in disease affected organs. Debora Konz Makino, Lab Head at CRELUX/WuXi AppTec, explains: ”Our long-standing collaboration with ESRF has greatly contributed to the success of most of our client's projects in the early stages of drug discovery. ESRF provides not only cutting edge infrastructure, but also excellent scientific support for X-ray data collection of biological macromolecules. We at CRELUX highly appreciate ESRF prompt and open communication.”
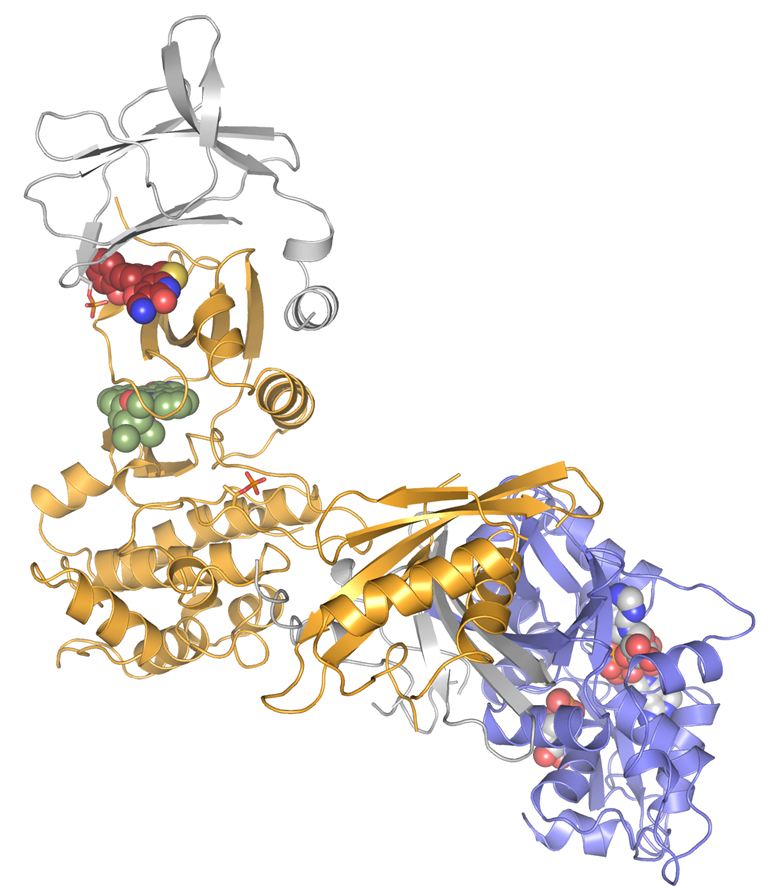 |
| The AMPK structure solved. Credits: CRELUX. |
 |
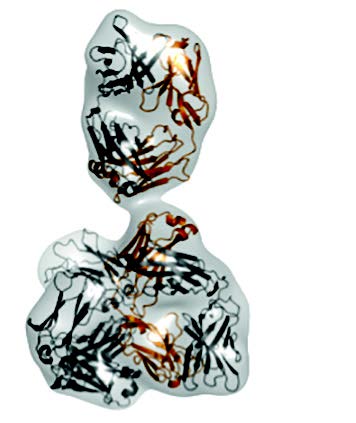 |
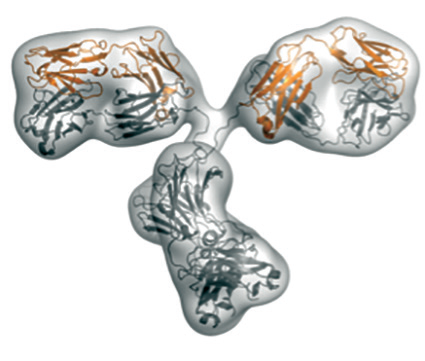 |
|
Envelope of the molecule calculated from the SAXS-data (top); conformation of the antibody as determined by X-ray crystallography (middle); Y-shaped conformation of a different antibody that fits to the SAXS-data (bottom). |
Company
Boehringer
Challenge
Antibodies are a vital part of our immune system. These proteins bind to specific antigen proteins on the surface of foreign bodies such as bacteria and viruses in order to neutralize or disarm them. Since each antigen has a different shape, it requires a different antibody to attach to it. By tailoring antibodies to attach to proteins responsible for specific diseases, pharmaceutical companies seek to develop drugs that minimise side-effects caused by antibodies binding to the wrong targets. Researchers at Boehringer have been studying a molecule in an antibody and they found that it was unusually compact as a single crystal. The next step was to obtain structural information of the molecule in solution.
Sample
The antibody molecule in solution.
Solution
Using Small Angle X-ray scattering, the team managed to compare the measured scattering curve with that calculated from the X-ray structure they had previously solved. The results confirmed that the compact conformation does not exist in solution. Instead, the molecule adopts a Y-shaped conformation, commonly known for antibodies.
Benefits
SAXS experiments can help pharmaceutical companies to double-check the results they get using their own characterization techniques. SAXS is also complementary of X-ray macromolecular crystallography, and, as proven here, can confirm or deny previous results.
Company
Teva Pharmaceuticals International GMBH, Jona (CH)
Challenge
Selexipag is a prostacyclin receptor agonist that causes vasodilation in pulmonary vasculature and is currently used to treat pulmonary arterial hypertension. This molecule is polymorphic, it exists in several polymorphs with distinct crystal forms and physical characteristics. Certain polymorphs of a drug can have beneficial characteristics for formulation such as chemical stability, shelf-life, dissolution profile and bioavailability. Teva wanted to patent particular polymorphs of selexipag for the treatment of arteriosclerosis, pulmonary hypertension and Raynaud's disease.
Sample
Solid state samples of selexipag forms IV and V.
Solution
Beamline ID22 is a high-resolution powder diffraction beamline. This beamline is routinely used for the characterisation of pharmaceuticals in crystalline or amorphous form, exploiting the high-resolution or hard-energy capabilities of the beamline, respectively.
Benefits
X-ray powder diffraction (XRPD) was used as a key analytical method to distinguish between polymorphs. The patent application concerns the synthesis of forms IV and V of the drug and their characterisation. XRPD along with other standard methods such as NMR and IR were used to characterise the polymorphs. XRPD provided a fingerprint to distinguish between the polymorphs. The crystal structure was calculated from the XRPD data, and these characteristics can also be used to precisely identify each polymorph.
 |
|
Chemical structure of selexipag. |
Reference
Solid state forms of Selexipag, Patent application: US 2018 0214446 A1, date: 2 Aug 2018.