- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Soft condensed matter
- Non-equilibrium collective dynamics in photo-excited lipid membranes
Non-equilibrium collective dynamics in photo-excited lipid membranes
All living systems are intrinsically out of thermal equilibrium. Their metabolism results in a multitude of local force centres on a molecular scale. ‘Active’ molecular transport in cells, motor proteins, and shape transformations of biological membranes are just some of the most prominent examples. From a fundamental point of view, the understanding of how a local energy release may change the properties of a soft biomolecular assembly remains elusive in many cases. We wanted to investigate how a system with long-range non-covalent interactions relaxes back to the equilibrium state after it has been driven out of equilibrium by external forces and the extent to which collective modes of many interacting biomolecules are involved.
For the important case of membranes, considered to be Nature’s most important interface, non-equilibrium fluctuations can be expected to be driven by active proteins or external forces. We have now shown that photo excitation of fluorophor-labelled lipids in a membrane can lead to a very characteristic collective response and have studied the pathway and time scales on which structural dynamics evolves.
 |
|
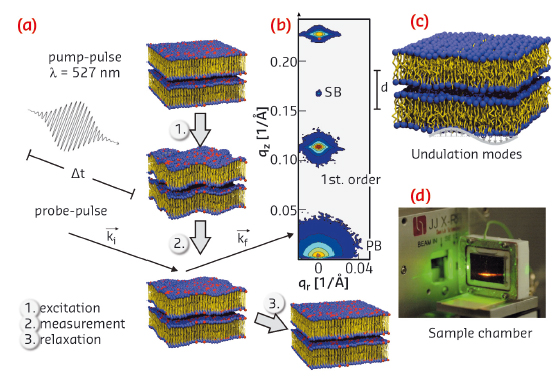
Fig. 33: a) Schematic of the laser pump/X-ray probe experiment at beamline ID09B on the multilamellar lipid stack in the fluid phase. The temporal evolution of membrane undulations was studied in response to a short pulse excitation, by recording the diffuse scattering pattern and line-shape analysis of the individual lamellar reflections. b) Example of a lamellar diffraction pattern with primary beam (PB), specular beam (SB), and the two first lamellar diffraction orders. c) From the two-dimensional distribution, the temporal evolution of the lateral and vertical correlation functions are extracted. d) Sample chamber for oriented Texas-Red labelled lipid multilayers fully immersed in solution. |
In the experiment carried out at beamline ID09B, we monitored the shape fluctuations in a stack of phospholipid bilayers mainly composed of the model lipid DOPC (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine), deposited on a quartz surface in solution. To excite the system, a short picosecond laser pulse was used, matched in wavelength to the absorption band of fluorescently labelled lipids (Texas-red), which were mixed into the membranes, as routinely used for optical fluorescence experiments. After energy uptake, the system’s response was probed by well defined picosecond X-ray pulses. The characteristic diffraction pattern of the membrane stack (non-specular diffuse scattering) was then recorded as a function of time delay, from a few picoseconds to several microseconds, before the procedure was repeated to gain sufficient statistics. Figure 33 summarises the experiment schematically.
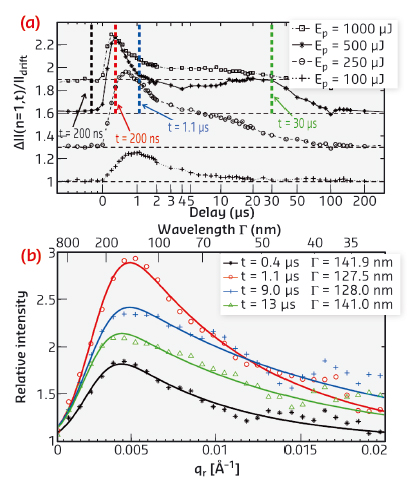
From the data, we extracted the temporal evolution of the height correlation functions describing the lipid bilayer undulations after excitation (Figure 34). The results indicate that pulsed laser illumination even at quite moderate peak intensities of about 105 W/cm2 leads to significant changes of the in plane membrane correlation length by up to 50% as well as the excitation of transient conformal undulation modes of a well defined lateral wavelength. The observed phenomena evolve on nano- to microsecond timescales after optical excitation, and can be described in terms of a modulation instability in the lipid multilamellar stack.
 |
|
Fig. 34: a) Evolution of the diffuse scattering intensity as a function of time after excitation. The increase reflects the transient increase in membrane undulations. b) A characteristic undulation mode with a wavelength band centred around 130 nm appears, reaching its maximum about 1 µs after excitation. |
In simple terms, the energy uptake at the molecular level leads to non-thermalised local vibrations which can be regarded as “local heat”, before the long-range undulation modes have equilibrated. In such a transient state, the lipid molecules tend to expand their inter-molecular distances, but on short time scales this motion is quenched. For compensation, the membranes buckle, opening up a faster relaxation mechanism than the thermal expansion of the entire system. The results may have important implications for many other optical fluorescence experiments, in which it is tacitly assumed that the supra-molecular structural state of the sample remains unchanged, apart perhaps from more or less negligible heat generation. After all, the excitation of embedded fluorophores in biomolecular assemblies is a very common tool in biomolecular and life science. Similar collective dynamic effects may thus occur in many photo labelled soft matter sample systems, effectively driving the structure away from thermal equilibrium.
Importantly, the non-equilibrium effects do not directly manifest themselves on the molecular length scale, but rather on mesoscopic scales via collective mechanisms. In the present example, local molecular energy uptake by absorption of photons in the membrane leads to peculiar long-range mesoscopic changes observable over microseconds before the sample has reached equilibrium again. The energy dissipation pathway involves a characteristic sequence of modes at length scales of a few hundred nanometres. We speculate that this may be a quite general phenomenon, not only applicable to optical excitation, but also to other excitation mechanisms and local force centres occurring in active states of biomolecular and soft matter.
Principal publication and authors
T. Reusch (a), D.D. Mai (a), M. Osterhoff (a), D. Khakhulin (b), M. Wulff (b) and T. Salditt (a), Physical Review Letters 111, 268101 (2013).
(a) Institut für Röntgenphysik, Georg-August-Universität Göttingen (Germany)
(b) ESRF



