- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structural biology
- Capturing a closed conformation of an ion channel
Capturing a closed conformation of an ion channel
Pentameric ligand-gated ion channels (pLGICs) are a major family of neurotransmitter receptors in the brain and its periphery that encompasses acetyl-choline, serotonine, glycine and γ-amino-butyric acid receptors. All these receptors share a common pentameric architecture with a large extracellular domain (ECD) and a transmembrane domain (TMD) that is composed of four a-helices, named M1 to M4, that form a funnel-shaped pore defined by the M2 helices of each of the subunits. pLGICs are dynamic proteins that couple neurotransmitter binding in the ECD to the opening of the ion channels embedded in their TMD. This coupling involves global conformational reorganisations, thereby generating discrete allosteric states including a basal closed state, an active open state stabilised by agonists and several desensitised closed states. A prerequisite for understanding the gating mechanism of pLGICs at the molecular level is to obtain structural information on each of these different allosteric states.
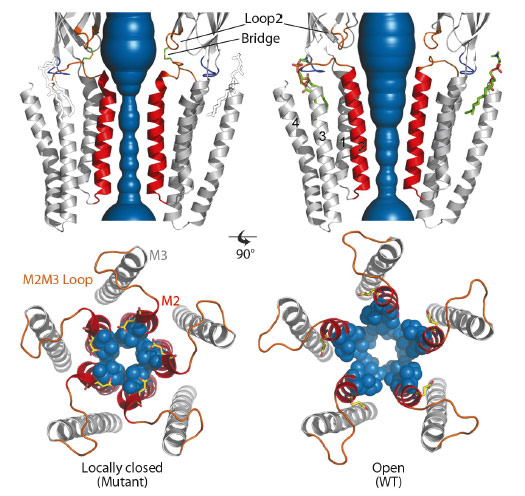
GLIC is a pH-gated bacterial homologue of the family [1] of which the structure has been solved in the presence of saturating concentrations of agonist protons in an open conformation (Figure 16) [2]. In the present work, our aim was to solve the X-ray structure of a closed conformation of GLIC. In order to do so, we generated four different cross-links between the ECD and the TMD. The four distinct mutants crosslink ECD loop 2 to four positions in the TMD M2-M3 loop. Electrophysiology experiments revealed that 3 out of 4 cross-linking mutations produce no current in their oxidised form suggesting that these channels are unable to open in the membrane context.
 |
|
Fig. 16: Comparison of the X-ray structures of a GLIC cross-linked mutant in the locally-closed conformation (left) and wild-type GLIC in the open conformation (right). The water accessible region of the pore of locally-closed and open forms is shown in blue. The lipid molecule seen only on the open structure is shown in green sticks and drawn on the locally-closed structure for reference. The bottom panels present top-views of the pores. M2 helices are coloured in red, and side chains of constricting residues are shown as blue spheres. |
We solved the structures of these four mutants by X-ray crystallography at 2.6-3.3 Å resolution. All four mutants display a common locally-closed conformation of the protein characterised by: i) an overall conformation very similar to the open form, with nearly identical conformations of the ECD, M1, M3 and M4 helices, ii) a locally-closed pore. The open to locally-closed conformational reorganisation involves an iris-like, symmetrical closure of the pore, where each M2 helix is bent and its upper part is tilted by 17° (Figure 16). In this process, the upper part of M2 detaches from M3 to interact with each other in a tightly packed bundle of five short helices that obstructs the pore. As a control, we checked that the X-ray structures of every mutant reduced with DTT prior to crystallisation returned to the known open conformation. In addition, we observe that a phospholipid binding site identified in the GLIC wild-type open structure is lost in all the locally-closed structures. For the reduced double mutants that crystallise in the open form, residual electron density in the Fo-Fc map clearly identified again the presence of phospholipids. This suggests that the binding of phospholipids could be part of the molecular determinants that stabilise the open form of the channel, in line with the possibility that some phospholipids may act as allosteric effectors of pLGICs. We performed similar cross-linking mutations on the human glycine a1 receptor and observed that they display similar phenotypes to that of the corresponding GLIC mutants. This suggests that our findings can be extended to eukaryotic pLGICs.
In addition, we showed that the locally-closed form can be adopted by a fully functional GLIC cross-linked mutant suggesting that these conformations may also occur in the membrane for a functional channel, outside of the crystal and detergent context. This idea is supported by molecular dynamics simulations showing that the locally-closed state is stable in a membrane environment, and also by cross-linking and SCAM experiments (substituted cysteine accessibility method) that are compatible with the locally-closed to open state transition. The locally-closed form we observed could thus correspond to a late intermediate in the course of activation or to an early intermediate in the course of desensitisation of GLIC. In our structures, the locally-closed form is also associated with a reshaping of the cavities located behind M2, known to mediate the effect of allosteric effectors, including general anaesthetics and alcohols. The locally-closed conformation might thus constitute a valid template for mechanistic investigations of the gating process and in the design of novel classes of pharmacological effectors targeted to neurotransmitter receptors.
Principal publication and authors
M. Prevost (a), L. Sauguet (a,b), H. Nury (a,b), C. Van Renterghem (a), C. Huon (a), F. Poitevin (b), M. Baaden (c), M. Delarue (b) and P.J. Corringer (a), N.S.M.B. 19, 642-9 (2012).
(a) Institut Pasteur, Groupe Récepteurs-Canaux, CNRS, Paris (France)
(b) Institut Pasteur, Unité de Dynamique Structurale des Macromolécules, CNRS, Paris (France)
(c) Laboratoire de Biochimie Théorique, CNRS, Univ Paris Diderot, Paris (France)
References
[1] N. Bocquet et al, Nature 445, 116-119 (2007).
[2] N. Bocquet et al, Nature 457, 111-114 (2009).



