- Home
- Users & Science
- Find a beamline
- Complex systems and biomedical sciences
- ID17 - Biomedical Beamline
- Microbeam Radiation Therapy (MRT)
Microbeam Radiation Therapy (MRT)
Microbeam Radiation Therapy (MRT) uses highly collimated, quasi-parallel arrays of X-ray microbeams of 50-600 keV, produced by 3rd generation synchrotron sources. The main features of highly brilliant Synchrotron sources are an extremely high dose rate and very small beam divergence. High dose rates are necessary to deliver therapeutic doses in microscopic volumes, to avoid spreading of the microbeams by cardiosynchronous movement of the tissues. The minimal beam divergence results in the obvious advantage of steeper dose gradients delivered to a tumour target, thus achieving a higher dose deposition in the target volume in fractions of seconds, with a sharper penumbra than that produced in conventional radiotherapy.
MRT research over the past 20 years has yielded many results from preclinical trials based on different animal models, including mice, rats, piglets and rabbits. Typically, MRT uses arrays of narrow (~25-75 micron-wide) microplanar beams separated by wider (100-400 microns centre to centre) microplanar spaces. Peak entrance doses of several hundreds of Gy are surprisingly well tolerated by normal tissues and at the same time show a preferential damage of malignant tumour tissues.
Comparisons between broad beam irradiations and MRT indicate a higher therapeutic index in the latter. A selective radiovulnerability of the tumour vasculature versus normal blood vessels by MRT and the involved cellular and molecular mechanisms are at the origin of these differential effects.
Materials and Methods
The production of such highly collimated, quasi-parallel arrays of X-ray microbeams ranging in energy from 50-600 keV is only feasible at a 3rd generation synchrotron source. The ESRF is currently among the most adequate sources for future clinical trials where the spreading of the microbeams due to cardiosynchronous movement of the tissues can be avoided by extremely rapid dose delivery. Because of the small beam divergence and the adequate photon spectrum (keV), a Multi Slit Collimator (MSC), inserted into the beam, produces and preserves steep dose gradients delivered to a tumour target within a fraction of seconds. The sharp dose gradients between peaks and valleys are preserved even after 15 cm of penetration of the microbeams in the depth of the tissue [1]. A 3D dose profile in a mouse head is presented in figure 1.
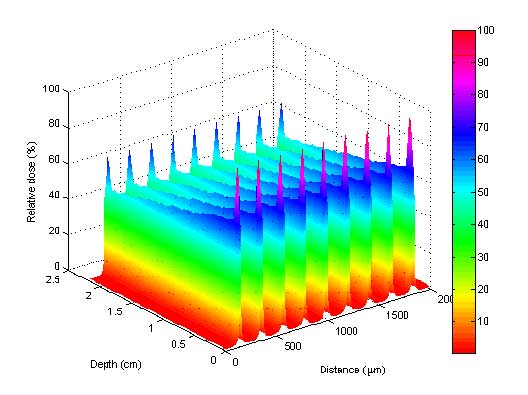
Figure 1: Monte Carlo simulated 3D dose profile of 9 parallel microbeams within a mouse head. The peaks at the entrance and at the exit show the increase in dose due to the presence of bone.
The wiggler source provides, at a distance of 40 m from the storage ring, a beam of about 40 mm in width and 1 mm in height. Thus, to irradiate a tumour volume of approximately 3 cm diameter, the target must be swept vertically through the beam in combination with a very fast shutter system [2] that defines exactly the upper and lower limit of the irradiated target zone.
The production of very regular microbeams is a crucial aspect for MRT. The development in instrumentation included the very first variable MSC (Archer collimator [3]), then the Tecomet MSC [4], and recently the advanced ESRF MSC (EMSC) produced from a solid tungsten carbide piece using new wire cutting techniques [5].
Historical overview in MRT and Summary of most important results
Spatial fractionation of ionizing radiation in the microscopic range was first reported in the sixties. A 25 µm-wide 22 MeV deuteron microbeam, used to simulate the effects of cosmic radiation (Curtis 1967)[6,7], failed to elicit cerebral damage in mice unless absorbed doses were over ~3000 Gy (Curtis 1963)[7,8]; the deuterons, however, reached only ~1.5 mm tissue depth.
Later, it appeared that "microbeam radiation therapy" (MRT), using arrays of microplanar, synchrotron-generated X-ray beams, safely delivered radiation doses to contiguous normal animal brains that were much higher than maximum doses tolerated by the same normal tissues of animals or patients from any standard millimetres-wide radiosurgical beam (Laissue et al, 1998[9]). Preclinical experiments began in 1995 at the ESRF and have been persued until today: Schweizer et al. 2000 [10]; Laissue et al, 1999, 2001 [11,12]; Blattmann et al, 2002 [13]; Bräuer-Krisch et al, 2005 [14], P. Regnard et al, 2008[15,16], and by R. Serduc 2008,2009 [17-19].
MRT delivers peak radiation doses up to fifty times higher than other radiosurgeries and spares fast-growing, immature tissues such as the duck brain in ovo (Dilmanian et al, 2001[20]) and the chick chorio-allantoic membrane in vitro (Blattmann et al, 2005[13,21]). In vivo, the cerebella of normal suckling Sprague-Dawley rat pups and of normal weanling piglets were irradiated by arrays of parallel, synchrotron-wiggler-generated X-ray microbeams in doses covering the MRT-relevant range (~50-600 Gy). Most animals developed normally over at least one year after irradiation (Laissue et al, 1999 [11,12], An example of a histological section of the hindbrain of the weaned piglets is shown in figure 2.
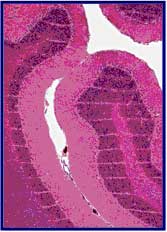
Figure 2: Microbeam irradiated normal CNS of weaned piglets (1.5cm x 1.5cm ~28 mm-wide beams ~210 mm on center, 625 Gy). The histological sections look normal, except for "stripes" due to the dropout of neuronal/astroglial nuclei. This sharp spatial fractionation is preserved throughout the cerebellum. No tissue necrosis, hemorrhage or demyelination was observed.
In preclinical trials, intracranial rat 9LGS and mouse EMT-6 carcinomas have been treated by variants of MRT; the growth of nearly every tumour was suppressed, at least temporarily, and many tumours were ablated (Laissue et al, 1998[9]; Dilmanian et al, 2002, 2003[22,23]; Smilowitz et al, 2006[24]). Even the extraordinarily radiation-resistant and fast-growing murine squamous cell carcinoma VII has been palliated by MRT (Miura et al, 2006[25]). For the intracerebral 9LGS, estimates of the therapeutic index of MRT versus broad-beam treatment indicate a ~5-fold advantage, with a normal tissue tolerance = 10-fold higher for peak microplanar versus seamless doses of radiation (Dilmanian et al, 2002[22]). In conventional radiotherapy, the effect of changing an irradiation parameter, e.g., the dose fractionation schedule, is predictable. Conversely, methods to predict the effect of varying MRT parameters such as array width and height, slit width, centre-to-centre spacing, number of ports, energy spectrum, dose microdistribution and schedules for temporal fractionation (Serduc et al, 2009[18]) or geometric adjustments (Bräuer-Krisch et al, 2005[14,26]) of multidirectional MRT are only beginning to be developed.
During the last 10 years, major progress has been made to better understand the underlying biological mechanisms, use the feature of MRT increasing the permeability of the tumour vasculature to inject additional drugs or dose enhancing agents as well as developments in Medical Physics to prepare clinical trials at the ESRF. Novel approaches for treating certain epilepsies show equally very promising results. Microbeams are used in fundamental research to create microscopic lesions to study mechanisms of certain neurological diseases.
References:
[1] E. Siegbahn, E. Brauer-Krisch, J. Stepanek, H. Blattmann, J. Laissue and A. Bravin Dosimetric studies of microbeam radiation therapy (MRT) with Monte Carlo simulations, Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 548 (2005) 54-58.
[2] M. Renier, T. Brochard, C. Nemoz and W. Thomlinson A White-beam Fast-Shutter for Microbeam Radiation Therapy at the ESRF, Nucl. Instrum. Methods A 479 (2002) 656-660
[3] D. Archer Collimator for producing an array of microbeams, United States Patent, 1998.
[4] E. Bräuer-Krisch, A. Bravin, E. Siegbahn, A., L. Zhang, J. Stepanek, H. Blattmann, D.N. Slatkin, J.O. Gebbers, M. Jasmin and J. Laissue Characterization of a tungsten/gas multislit collimator for microbeam radiation therapy at the European Synchrotron Radiation Facility., Review of scientific instruments 76 (2005) 1-7.
[5] E. Bräuer-Krisch, H. Requardt, T. Brochard, G. Berruyer, M. Renier, J. Laissue and A. Bravin New technology enables precision multi slits collimators for MRT (Microbeam Radiation Therapy), Accepetd Review of Scientific Instruments (2009).
[6] H. Curtis The use of deuteron microbeam for simulating the biological effect of heavy cosmic-ray particles, Radiat Res supplement 7 (1967) 250-257.
[7] H. Curtis The microbeam as a tool in radiobiology, Adv Biol Med Phys 175 (1963) 207-224.
[8] H.J. Curtis The interpretation of microbeam experiments for manned space flight, Radiat Res Suppl 7 (1967) 258-264.
[9] J.A. Laissue, G. Geiser, P.O. Spanne, F.A. Dilmanian, J.O. Gebbers, M. Geiser, X.Y. Wu, M.S. Makar, P.L. Micca, M.M. Nawrocky, D.D. Joel and D.N. Slatkin Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays, Int J Cancer 78 (1998) 654-660.
[10] P.M. Schweizer, P. Spanne, M. Di Michiel, U. Jauch, H. Blattmann and J.A. Laissue Tissue lesions caused by microplanar beams of synchrotron-generated X-rays in Drosophila melanogaster, Int J Radiat Biol 76 (2000) 567-574.
[11] J.A. Laissue, N. Lyubimova, H.P. Wagner, D.W. Archer, D.N. Slatkin, M. Di Michiel, C. Nemoz, M. Renier, E. Bräuer-Krisch, P.O. Spanne, J.-O. Gebbers, K. Dixon and H. Blattmann Microbeam radiation therapy, Proc. Of SPIE, Denver, USA, 1999, pp. 38-45.
[12] J.A. Laissue, H. Blattmann, D. Michiel, D.N. Slatkin, N. Lyubimova, R. Guzman, A. Zimmermann, S. Birrer, T. Bey, P. Kircher, R. Stettler, R. Fatzer, A. Jaggy, H.M. Smilowitz, E. Bräuer-Krisch, A. Bravin, G. Le Duc, C. Nemoz, M. Renier, W. Thomlinson, J. Stepanek and H.P. Wagner The weanling piglet cerebellum: a surrogate for tolerance to MRT (microbeam radiation therapy) in pediatric neuro-oncology, Proc. of SPIE, Washington, 2001, pp. 65-73.
[13] H. Blattmann, W. Burkard, V. Djonov, M. DiMichiel, E. Brauer, J. Stepanek, A. Bravin, J.-O. Gebbers and J. Laissue Microbeam irradiation in the chorio-allantoic membrane (CAM) of chicken embryo, Strahlenther. Onkol 178 (2002) 118.
[14] E. Bräuer-Krisch, H. Requardt, P. Regnard, S. Corde, E. Siegbahn, G. Leduc, T. Brochard, H. Blattmann, J. Laissue and A. Bravin New irradiation geometry for microbeam radiation therapy, Phys Med Biol 50 (2005) 3103-3111.
[15] P. Regnard, E. Bräuer-Krisch, I. Tropes, J. Keyrilainen, A. Bravin and G. Le Duc Enhancement of survival of 9L gliosarcoma bearing rats following intrcerbral delivery of drugs in combination with microbeam radiation therapy, Europ J Radiol 68 (2008) S 151-155.
[16] P. Regnard, G.L. Duc, E. Bräuer-Krisch, I. Tropres, E.A. Siegbahn, A. Kusak, C. Clair, H. Bernard, D. Dallery, J.A. Laissue and A. Bravin Irradiation of intracerebral 9L gliosarcoma by a single array of microplanar x-ray beams from a synchrotron: balance between curing and sparing, Phys Med Biol 53 (2008) 861-878.
[17] R. Serduc, A. Bouchet, E. Bräuer-Krisch and G. Le Duc Microbeam radiation therapy parameters optimization for rat brain tumors palliation. Influence of the microbeam width at constant valley dose, Submited Phys Med Biol (2009).
[18] R. Serduc, E. Brauer-Krisch, A. Bouchet, L. Renaud, T. Brochard, A. Bravin, J.A. Laissue and G. Le Duc First trial of spatial and temporal fractionations of the delivered dose using synchrotron microbeam radiation therapy, J Synchrotron Radiat 16 (2009) 587-590.
[19] R. Serduc, B. Lemasson and A. Bouchet Rat brain tumor pallation by microbeam radiation therapy, the vascular component., Under preparation (2009).
[20] F.A. Dilmanian, G.M. Morris, G. Le Duc, X. Huang, B. Ren, T. Bacarian, J.C. Allen, J. Kalef-Ezra, I. Orion, E.M. Rosen, T. Sandhu, P. Sathe, X.Y. Wu, Z. Zhong and H.L. Shivaprasad Response of avian embryonic brain to spatially segmented x-ray microbeams, Cell Mol Biol 47 (2001) 485-493.
[21] H. Blattmann, J.-O. Gebbers, E. Brauer-Krisch, A. Bravin, G. Le Duc, W. Burkard, D. Michiel, V. Djonov, D.N. Slatkin, J. Stepanek and J. Laissue Applications of synchrotron X-rays to radiotherapy, Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 548 (2005) 17-22.
[22] F.A. Dilmanian, T.M. Button, G. Le Duc, N. Zhong, L.A. Pena, J.A. Smith, S.R. Martinez, T. Bacarian, J. Tammam, B. Ren, P.M. Farmer, J. Kalef-Ezra, P.L. Micca, M.M. Nawrocky, J.A. Niederer, F.P. Recksiek, A. Fuchs and E.M. Rosen Response of rat intracranial 9L gliosarcoma to microbeam radiation therapy, Neuro-oncol 4 (2002) 26-38.
[23] F.A. Dilmanian, G.M. Morris, N. Zhong, T. Bacarian, J.F. Hainfeld, J. Kalef-Ezra, L.J. Brewington, J. Tammam and E.M. Rosen Murine EMT-6 carcinoma: high therapeutic efficacy of microbeam radiation therapy, Radiat Res 159 (2003) 632-641.
[24] H.M. Smilowitz, H. Blattmann, E. Bräuer-Krisch, A. Bravin, M.D. Michiel, J.O. Gebbers, A.L. Hanson, N. Lyubimova, D.N. Slatkin, J. Stepanek and J.A. Laissue, Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas, J Neuro-oncol 78 (2006) 135-143.
[25] M. Miura, H. Blattmann, E. Bräuer-Krisch, A. Bravin, A.L. Hanson, M.M. Nawrocky, P.L. Micca, D.N. Slatkin and J.A. Laissue Radiosurgical palliation of aggressive murine SCCVII squamous cell carcinomas using synchrotron-generated X-ray microbeams, Br J Radiol 79 (2006) 71-75.
[26] Effets de radiothérapie microfaisceaux rayonnement synchrotron X sur la micro-vascularisation cérébrale chez la souris. R.Serduc, P. Vérant, R.Farion, J.C. Vial, C. Rémy, E. Bräuer, A. Bravin, B. Van der Sanden
[27] A differential in vivo 2 photon microscopy method for quantitative imaging of dye extravasation in mouse cortex Pascale Vérant, Jean Claude Vial, Raphaël Serduc, Clement Ricard, Jonathan Coles, Chantal Rémy, Elke Bräuer, Alberto Bravin, Boudewijn van der Sanden
[28] E. A. Siegbahn, J. Stepanek, E. Bräuer-Krisch, and A. Bravin ”Determination of dosimetrical quantities used in microbeam radiation therapy (MRT) with Monte Carlo simulations”, Medical Physics,Vol. 33, No.9, 3248-3259, 2006
[29] J. Spiga, E. A. Siegbahn, E. Bräuer-Krisch, P. Randaccio, A. Bravin “The GEANT4 toolkit for microdosimetry calculations: Applicationto microbeam radiation therapy (MRT)”, Medical Physics, Vol. 34, No. 11, November 2007
[30] Raphael Serduc, Yohan van de Looij, Gilles Francony, Olivier Verdonck, Boudewijn van der Sanden, Jean Laissue, Regine Farion, Elke Bräuer-Krisch, Erik Albert Siegbahn, Alberto Bravin, Yolanda Prezado, Christoph Segebarth, Chantal Remy and Hana Lahrech “Characterization and quantification of cerebral edema induced by synchrotron x-ray microbeam radiation therapy” Phys Med Biol. 2008 Mar 7;53(5):1153-66
[31] J.C. Crosbie, I. Svalbe, S.M. Midgley, N. Yagi, P.A. Rogers and R.A. Lewis A method of dosimetry for synchrotron microbeam radiation therapy using radiochromic films of different sensitivity, Phys Med Biol 53 (2008) 6861-6877.
[32] P. Vérant, J. Vial, R. Serduc, C. Ricard, J. Coles, C. Rémy, B. van der Sanden, E. Bräuer-Krisch, and A. Bravin, "A differential in vivo two photon micrcoscopy method for quantitative imaging of dye extravasation in mouse cortex," in Biomedical Optics, Technical Digest (CD) (Optical Society of America, 2006), paper ME27
[33] Regnard P, Le Duc G, Bräuer-Krisch E, Irene Tropres, Erik Albert Siegbahn, Audrey Kusak, Charlotte Clair, Helene Bernard, Dominique Dallery, Jean A Laissue “Irradiation of intracerebral 9L gliosarcoma by a singlearray of microplanar x-ray beams from asynchrotron: balance between curing and sparing” Phys. Med. Biol. 53 (2008) 861–878 doi:10.1088
[34] E. Bräuer-Krisch, A. Bravin, M. Lerch, A. Rosenfeld, J. Stepanek, M. Di Michiel, and J. A. Laissue, “MOSFET dosimetry for microbeam radiation therapy at the European Synchrotron Radiation Facility,” Med. Phys. 30, 583-589 (2003).
[35] E.A.Siegbahn, E. Bräuer-Krisch, A. Bravin, H. Nettelbeck, M.L.F.Lerch and A.B. Rosenfeld “Mosfet Dosimetry with high spatial resolution in intense synchrotron-generated x-ray microbeams” Med. Physics 36 (4) 1128-1137, April 2009
[36] M. Ptaszkiewicza, E. Bräuer-Krisch, M. Klosowski, L. Czopyk, P. Olko “
[37] Elisabeth Schültke, Bernhard H.J. Juurlink , Khalid Ataelmannan , Jean Laissue ,Hans Blattmann , Elke Bräuer-Krisch , Alberto Bravin , Joanna Minczewskaf, Jeffrey Crosbie , Hadi Taherian , Evan Frangou , Tomasz Wysokinsky ,L. Dean Chapmana, Robert Griebel , Daryl Fourney “Memory and survival after microbeam radiation therapy” EJR 2008, 68(3 Suppl): 142-6.
[38] “Monte Carlo code comparison of dose delivery prediction for Microbeam Radiation Therapy” Felici M De., Siegbahn E.,Spiga J., Hanson A.L., Felici R., Ferrero C., Tartari A. , Gambaccini M., Keyrilainen J., Brauer-Krisch E., Randaccio P., Bravin A. third McGill international Workshop Journal of Physics Conference series 102 (2008) 012005
[39] Raphael Serduc, Thomas Christen, Jean Laissue, R´egine Farion1,Audrey Bouchet, Boudewijn van der Sanden, Christoph Segebarth, Elke Bräuer-Krisch, G´eraldine Le Duc, Alberto Bravin, Chantal R´emy and Emmanuel L Barbier “Brain tumor vessel response to synchrotron microbeam radiation therapy: a short-termin vivo study”, Phys. Med. Biol. 53 (2008) 3609–3622
[40] E. A. Siegbahn,_ E. Bräuer-Krisch, and A. Bravin, H. Nettelbeck, M. L. F. Lerch, and A. B. Rosenfeld “MOSFET dosimetry with high spatial resolution in intense synchrotron-generated x-ray microbeams” 1128-1137 Med. Phys. 36 4 April 2009
[41] E. Bräuer-Krisch, H. Requardt, T. Brochard, G. Berruyer, M. Renier, LJ.A. Laissue, A. BravinE. Bräuer-Krisch et al. “New Technology enables high precision Multi Slit Collimators for MRT ” RSI 80, 074301 (2009) (38 cit.)
[42] Raphael Serduc, Elke Bräuer-Krisch, Audrey Bouchet, Luc Renaud, Thierry Brochard, Alberto Bravin, Jean Laissue and Geraldine Le Duc “First trial of spatial and temporal fractionations of the delivered dose using synchrotron microbeam radiation therapy” Journal of Synchrotron Radiation ISSN 0909-0495 Accepted 2 April 2009
[43] R. Serduc, E. Bräuer-Krisch, E.A Siegbahn, A. Bouchet, B. Pouyatos, R. Carron, N. Pannetier, L. Renaud, G. Berruyer, C. Nemoz, T. Brochard, C. Rémy, E. Barbier, A. Bravin, G. Le Duc, A. Depaulis, F. Estève, J. Laissue “High-Precision Radiosurgical Dose Delivery by Interlaced Microbeam Arrays of High-Flux Low-Energy Synchrotron X-Rays “PLoS
[44] E. Schültke, K. Ataelmanan, H. Blattmann, A. Bravin, Elke Bräuer-Krisch, D. Fourney, R. W. Griebel, J. A. Laissue, N. Sidhu, E. Simko, B. H. J. Juurlink “Experimental radiotherapy for malignant brain tumors: The glutathione synthesis inhibitor BSO and MRT”
[45] Boudewijn van der Sanden, Elke Bräuer-Krisch, Eric Siegbahn, Clément Ricard, Catherine Massart, Jean-Claude Vial, Jean Laissue “Tolerance of small arteries for high dose X-ray microplanar beams depends on smooth muscle cells of the normal media” Accepted by International Journal of Radiation Oncology, Biology, Physics. Ms. No. ROB-D-09-01479R2
[46] A.T. Abdul Rahman, D.A. Bradley, S.J. Doran, 1, Brochard Thierry, Elke Braeuer-Krisch, A. Bravin “The Thermoluminescence Response of Ge-doped Silica Fibres for Synchrotron Microbeam radiation therapy Dosimetry” 11th int. Symposium on Radiation Physics, 20-25 Sept. Melbourne, Australia
[47] Elke Bräuer-Krisch, R. Serduc, E. A. Siegbahn, G. Le Duc, Y. Prezado, A. Bravin, H. Blattmann, J. A. Laissue ”Effects of pulsed spatial fractionated microscopic beams on normal and tumoral brain tissue” Mutat Res 2010; 704:160-6 (91 cit)
[48] S. J Doran, T. Brochard, J. Adamovics, N. Krstajic and E. Bräuer-Krisch “An investigation of the potential of optical computed tomography for imaging of synchrotron-generated x-rays at high spatial resolution” Phys. Med. Biol. 55 (2010) 1531–1547
[49] E. Bräuer-Krisch, E.A. Siegbahn and A. Bravin GafChromic® Film Measurements for Microbeam Radiation Therapy (MRT), IFMBE Proceedings 25 (2009).
[50] Elke Bräuer-Krisch A. Rosenfeld, M. Lerch, M. Petasecca, M. Akselrod, J. Sykora, J. Bartz, M. Ptaszkiewicz, P. Olko, A. Berg, M. Wieland, S. Doran, T. Brochard, A. Kamlowski, G. Cellere, A. Paccagnella, E. Siegbahn, Y. Prezado, I. Martinez-Rovira, A. Bravin, L. Dusseau, P. Berkvens “Potential High Resolution Dosimeters For MRT” 2010, MRT AIP Conf. Proc. 1266 89–97
[51] Y. Prezado, J.F. Adam, P. Berkvens, I. Martinez-Rovira, G. Fois, S. Thengumpallil, M. Edouard, M. Vautrin, P. Deman, E. Bräuer-Krisch, M. Renier, H. Elleaume, F. Estève and A. Bravin “Synchrotron Radiation Therapy from a Medical Physics point of view” AIP Conference Proceedings 07/2010; 1266(1):101-106.
[52] J. Bartz, J. Sykora, E. Bräuer-Krisch, M. Akselrod “Imaging
[53] S. Sarun, S. GilDuran, W. Fauquette, E. Diserbo, E. Bräuer-Krisch, Y. Prezado “A kinetic in vitro survival response of glioma cell lines using Synchrotron Minibeam Radiation Therapy” Estro March 2010
[54] R. Serduc, A. Bouchet, E. Bräuer-Krisch, J. Laissue, S. Sarun, A. Bravin, C. Fonta, L. Renaud, J. Boutonnat, E.A Siegbahn, F. Estève, G. Le Duc “Synchrotron microbeam radiation therapy for rat brain tumor palliation—influence of the microbeam width at constant valley”Phys. Med. Biol. 54 (2009) 6711–6724 (46 cit)
[55] A. Bouchet, B. Lemasson, G. LeDuc, C. Maisin, , Elke Bräuer-Krisch et al. ‘Preferential effect of synchrotron microbeam radiation therapy on intracerebral 9L gliosarcoma vascular networks” Int. J. Radiation Oncology Biol. Phys., Vol. 78, No. 5, pp. 1503–1512, 2010 (63 cit)
[56] A.T. Abdul Rahman, E. Bräuer-Krisch, T. Brochard, J. Adamovics, S.K. Clowes, D. Bradley and S.J. Doran Sophisticated test objects for the quality assurance of optical computed tomography scanners, Physics in medicine and biology 56 (2011) 4177-4199
[57] M. Lerch, H. Nettelbeck, H. Requardt, A. Cullen, E. Baloglow, E. Bräuer-Krisch, A. Bravin, M. Reinhard, R. Siegele, V. Perevertaylo and A. Rosenfeld “Multichannel silicon detectors for on-line synchrotron X-ray microbeam radiation dosimetry”, 10th International Conference on Synchrotron Radiation Instrumentation, September, Melb. 2009, 10th International Conf. on Synchrotron Radiation Instrumentation, September, Melb. 2009 (2009).
[58] H. Requardt, A. Bravin, Y. Prezado, E. BräuerKrisch, M. Renier et al.” The Clinical Trials Program at the ESRF Biomedical Beamline ID17: Status and Remaining Steps” AIP Conf. Proc. 1234, 161 (2010); doi: 10.1063/1.3463164
[59] S. Sabatasso, J.A. Laissue, R. Hlushchuk, E. Bräuer-Krisch, A. Bravin, H. Blattmann, K. Michaud and V. Djonov Vascular toxicity of microbeam irradiation depends on the stage of capillary maturation, Cardiovascular Research 87 (2010) S99-S99.
[60] R. Griffin, N. Koonce, R.P.M. Dings, Siegel, E, E. Moros and , Elke Bräuer-Krisch, Corry, PM “Microbeam radiation therapy alters microvascular architecture and tumor oxygenation and is enhanced by anti-angiogenic peptide therapy”, Radiation Research, Volume 177, Issue 6 (June 2012) (20 cit.)
[61] B. Pouyatos, R. Serduc, M. Chipaux, T. Chabrol, , Elke Bräuer-Krisch, C. Nemoz, H. Mathieu, O. David, L. Renaud, Y. Prezado, J. Laissue, F. Esteve, S. Charpier and A. Depaulis Synchrotron x-ray interlaced microbeams suppress paroxysmal oscillations in neuronal networks initiating generalized epilepsy., Neurobiology of Disease 51 (2013) 152–160.
[62] R.W. Smith, J. Wang, E. Schultke, L.E.J. Lee, C.B. Seymour, E. Bräuer-Krisch, J. Laissue and C.E. Mothersill “Proteomic changes in the rat brain induced by homogenous irradiation and by the bystander effect resulting from high energy synchrotron X-ray microbeams”, Int J Radiat Biol. 2013 Feb;89(2):118-27
[63] M. Petasecca, A. Cullen, I. Fuduli, A. Espinoza, C. Porumb, C. Stanton, A. H. Aldosari, E. Bräuer-Krisch, H. Requardt, A. Bravin, V. Perevertaylo, A. B. Rosenfeld, and M. L. F. Lerch, “X-Tream: a novel dosimetry system for Synchrotron Microbeam Radiation Therapy,” J. Instrum., vol. 7, no. 07, pp. P07022–P07022, Jul. 2012
[63] Le Duc, G ; Miladi, I; Alric, C; Mowat, P ; Brauer-Krisch, E; Bouchet etal “Toward an Image-Guided Microbeam Radiation Therapy Using Gadolinium-Based Nanoparticles” ACS Nano Volume: 5 Issue: 12 Pages: 9566-9574 2011 (95 cit.)
[64] Elke Bräuer-Krisch, C. Nemoz, Th. Brochard, G. Berruyer, M. Renier, B. Pouyatos, R. Serduc, ’The preclinical set-up at the ID17 biomedical beamline to achieve high local dose deposition using interlaced microbeams” Ref.: Ms. No. JPCS181 Journal of Physics: Conference Series SRI 2012
[65] J.A. Laissue , S. Bartzsch, H. Blattmann, , Elke Bräuer-Krisch, A. Bravin, D. Dalléry, V. Djonov, A. L. Hanson, J.W. Hopewell, B. Kaser-Hotz, J. Keyriläinen, P. Laissue, M. Miura, R. Serduc, A. E. Siegbahn, D. N. Slatkin “Response of the rat spinal cord exposed to X-ray microbeams” Radiother Oncol 106, 1 (2013) 106-11
[66] Carmel Mothersill, Cristian Fernandez-Palomo, Jennifer Fazzar, Richard Smith, Elisabeth Schültke, Elke Bräuer-Krisch, Jean Laissue, Christian Schroll, Colin Seymour “Transmission of signals from rats receiving high doses of microbeam radiation to cage mates: an inter-mammal bystander effect Dose-Response 12:72-92, 2014
[67] Romanelli P, Fardone E, Battaglia G, Bräuer-Krisch E, Prezado Y, et al. (2013)Synchrotron-Generated Microbeam Sensorimotor Cortex Transections Induce Seizure Control without Disruption of Neurological Functions.PLoS ONE 8(1):e53549.doi:10.1371/journal.pone.0053549
[68] Elisabeth Schultke1, Michael Trippel, Bräuer-Krisch E, Michel Renier, Stefan Bartzsch,Herwig Requardt, Mate´ D. D brossy, Guido Nikkhah “Pencilbeam Irradiation Technique for Whole Brain Radiotherapy: Technical and Biological Challenges in a Small Animal Model”PLOS ONE 1 January 2013 | Volume 8 | Issue 1 | e54960
[69] Stefan Bartzsch, Elke Bräuer-Krisch, Michael Lerch, Uwe Oelfke “Influence of Polarisation and a source model for dose calculation in MRT”, accepted Medical Physics 2014
[71] Simon Doran, A.T. Abdul Rahman, Elke Bräuer-Krisch, Thierry Brochard, John Adamovics, Andrew Nisbet, David Bradley “Establishing the suitability of quantitative optical CT microscopy of PRESAGE® radiochromic dosimeters for the verification of synchrotron microbeam therapy” submitted to PMB 2013
[72] Imen Miladi, Christophe Alric, Sandrine Dufort, Pierre Mowat, Aurélie Dutour, Elke Bräuer-Krisch, Jean-Luc Coll, Marie Dutreix, François Lux, Rana Bazzi, Claire Billotey, Marc Janier, Pascal Perriat, Géraldine Le Duc, 5 Stéphane Roux, Olivier Tillement “The in vivo radiosensitizing effect of gold nanoparticles based MRI contrast agents” SMALLVolume 10, Issue 6, pages 1116–1124, March 26, 2014
[73] Bouchet A, Lemasson B, Christen T, Potez M, Rome C, Coquery N, Le Clec'h C, Moisan A, Bräuer-Krisch E, Leduc G, Rémy C, Laissue JA, Barbier EL, Brun E, Serduc R. “Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain”, Radiother Oncol. 2013 Jul;108(1):
[74] Fernandez-Palomo C, Bräuer-Krisch E, Trippel M, Schroll C, Requardt H, Bartzsch S, Nikkhah G, Schültke E. DNA double strand breaks in the acute phase after synchrotron pencilbeam irradiation. JINST. 2013,
[75] Simon Doran, Thierry Brochard, John Adamovics, Nicola Krstajic and Elke Bräuer-Krisch “An investigation of the potential of optical computed tomography for imaging of synchrotron-generated x-rays at high spatial resolution” Phys. Med. Biol. 55 (2010) 1531-1547
[76] Bouchet Audrey, Sakakini Nathalie, El Atifi Michèle, Le Clec’h Céline, Elke Bräuer-Krisch, Moisan Anaïck, Deman Pierre, Rihet Pascal, Le Duc Géraldine, Pelletier Laurent “Early gene expression analysis in 9L orthotopic tumor-bearing rats identifies immune modulation as a hallmark of response to synchrotron microbeam radiation therapy” PLoS One. 2013 Dec 31;8(12)
[77] P. Berkvens , E. Bräuer-Krisch, T. Brochard, H. Requardt, C. Nemoz , M. Renier , P. Fournier Menyhert Kocsis Highly robust, high intensity white synchrotron X-ray beam monitor, ESRF, IEEE NSS MIC conference , Seoul 2013
[78] Iwan Cornelius, Susanna Guatelli, Pauline Fournier, Jeffrey C. Crosbie, Manuel Sanchez del Rio, Elke Bräuer-Krisch, Anatoly Rosenfeld and Michael Lerch “Benchmarking and Validation of a Geant4-SHADOW Monte Carlo Simulation for Dose Calculations in Microbeam Radiation Therapy” Journal of Synchrotron Radiation, 21(3), 518-528, 2014
[79] Bouchet, A., Sakakini, N. , El Atifi,M. ,Le Clec’h, C. , Bräuer-Krisch, E., Rogalev, L, Laissue, J.A., Rihet, P., Le Duc, G. ,Pelletier, L. “Identification of AREG and PLK1 pathway modulation as a potential key of the response of intracranial 9L tumor to microbeam radiation therapy”, International Journal of Cancer 2014
[80] Cristian Fernandez-Palomo, Carmel Mothersill, C., Bräuer-Krisch, E., E. , Jean Laissue, J.A., Seymour, C. , Schültke, E. g-H2AX as a marker for dose deposition in the brain of Wistar rats after Synchrotron Microbeam Radiation PLOS One 2014 (cited 10)
[81] Dan Sporea,D., Mihai, L.,Sporea, A. Alin Lixandru,A., Bräuer-Krisch, E., “Investigation of UV optical fibers under synchrotron irradiation” accepted Optics Express 2015
[82] S. Girst, C. Marx, E. Bräuer-Krisch, A. Bravin, S. Bartzsch, U. Oelfke, C. Greubel, J. Reindl, C. Siebenwirth, O. Zlobinskaya, G. Multhoff, G. Dollinger, T.E. Schmid, J.J. Wilkens “Improved normal tissue protection by proton and x-ray microchannels compared to homogeneous field irradiation” EJMP –.” Medica Physica, 2015 Special Issue SYRA3
[83] M. Donzelli, E. Bräuer-Krisch, C. Nemoz, T. Brochard, U. Oelfke “Conformal image-guided Microbeam Radiation therapy at the ESRF biomedical Beamline ID17” Medical physics, 2016
[84] E. Bräuer-Krisch et al “Medical Physics aspects in Synchrotron Radiation Therapies: MRT (MicrobeamRadiationTherapy) and SSRT (Synchrotron Stereotactic RadioTherapy) ” EJMP – .” Medica Physica, 2015 Special Issue SYRA3
[85] Géraldine Le Duc, Stéphane Roux, Paruta-Tuarez, A., Sandrine Dufort, Elke Bräuer-Krisch, Mariais, A., Truillet, C, Lucie Sancey, Pascal Perriat, François Lux, Olivier Tillement “” Cancer Nanotechnology, 2014 5:4, 1-14
[86] Sandrine Dufort, Géraldine Le Duc, Murielle Salome, Valerie Bentivegna, Lucie Sancey, Elke Bräuer-Krisch, Herwig Requardt, François Lux, Jean-Luc Coll, Pascal Perriat, Stéphane Roux, Olivier Tillement “The high radiosensitizing efficiency of a trace of gadolinium based nanoparticles in the tumor” JACS, 2015
[87] Cristian Fernandez-Palomo, Elke Bräuer-Krisch, Jean Laissue, Hans Blattmann, Colin Seymour, Elisabeth Schültke and Carmel Mothersill “Use of Synchrotron medical microbeam irradiation to investigate radiation-induced bystander and abscopal effects in vivo.” Medica Physica, 2015 ” EJMP – Special Issue SYRA3
[88] Studer F, Serduc R, Pouyatos, Chabrol, Bräuer-Krisch, Donzelli, Nemoz, Laissue JA, Estève F, Depaulis A “Synchrotron X-ray microbeams: A promising tool for drug-resistant epilepsy treatment. Medica Physica, accepted 2015 ” EJMP – .” Medica Physica, 2015 Special Issue SYRA3
[89] M. A. Grotzer, E. Schültke, E. Bräuer-Krisch, J. A. Laissue “Microbeam Radiation Therapy: Clinical Perspectives” Physica Medica European Journal of Medical Physics .” Medica Physica, 2015
[90] Bartzsch, S., Lott, J., Welsch, K. Bräuer-Krisch, Oelfke, U. “Micrometer-resolved Film Dosimety Using a Microscope in Microbeam Radiation Therapy (MRT) 2015 Medical Physics, Special Issue SYRA3
[91] Pantaleo Romanelli, Erminia Fardone, Giuseppe Battaglia, Elke Bräuer-Krisch, Herwig Requardt, Geraldine Le Duc, Alberto Bravin Microradiosurgical cortical transections generated by synchrotron radiation, ” Physica Medica European Journal of Medical Physics 2015, Special Issue SYRA3
[92] Crosbie, J., Fournier, P. Bartzsch, S., Donzelli, M., Cornelius, I., Stevenson, A. Requardt, H. Bräuer-Krisch, E. ‘Energy spectra considerations for synchrotron radiatherapy trials on the ID17-biomedical beamline at the European Synchrotron Radiation Facility ‘ Journal of Synchrotron Radiation, 2015
[93] “Assessment of optical CT as a future QA tool for synchrotron x-ray microbeam therapy" by McErlean, Ciara; . Bräuer-Krisch, Adamovics, John; Doran, Simon Article reference: PMB-103050.R1 Physics in Medicine and Biology Dec. 2015
[94] “In vivo pink-beam imaging and fast alignment procedure for rat brain tumor radiation therapy” Christian Nemoz, Astrid Kibleur, Jean Noel Hyacinthe, Gilles Berruyer, Thierry Brochard, Elke Bräuer-Krisch, Géraldine Le Duc, Emanuel Brun, Helene Helleaume and Raphaël Serduc , Journal of Synchrotron Radiation 23(1) · January 2016
[95] Better efficacy of synchrotron spatially fractionated radiotherapy than uniform radiotherapy on glioma” Bouchet Audrey, Bräuer-Krisch Elke, Yolanda Prezado, Michèle El Atifi, Rogalev Léonid, Le Clec’h Céline, Laissue Jean, Pelletier Laurent, Le Duc Géraldine, International journal of Radiation Oncolog, June 2016
[96] Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo” Daniel Broennimann, Audrey Bouchet, Christoph Schneider, Marine Potez , Raphaël Serduc, Elke Bräuer-Krisch, Werner Graber, Stephan von Gunten, Jean Albert Laissue and Valentin Djonov, Scientific reports, 2016
[97] "The High Radiosensitizing Efficiency of a Trace of Gadolinium-Based Nanoparticles in Tumors" by Dufort S, Le Duc G, Salomé M, Bentivegna M, Sancey L, Bräuer-Krisch E., Requardt H, Lux F, Coll JL, Perriat P, Roux O, Tillement O. Scientific Reports
[98] Fernandez-Palomo C., Schültke E , Bräuer-Krisch E, Laissue JA, Blattmann H, Seymour C, Mothersill C. "Investigation of abscopal and bystander effects in immunocompressed mice after exposure to pencil beam and microbeam synchrotron radiation”, Health Physics Journal 2016 Aug;111(2):149-59
[99] “X-Tream Quality Assurance in Synchrotron X-Ray Microbeam Radiation Therapy”
Pauline Fournier, Iwan Cornelius, Mattia Donzelli, Herwig Requardt, Christian Nemoz, Marco Petasecca, E. Bräuer-Krisch, Anatoly Rosenfeld and Michael Lerch, 2016, Journal of Synchrotron Radiation.
[100] “Synchrotron X-ray microtransections: a non invasive approach for epileptic seizures arising from eloquent cortical areas” B.Pouyatos, C. Nemoz, T. Chabrol, M. Potez, E.Bräuer-Krisch, L. Renaud, K. Pernet-Gallay, F. Esteve, P. Kahane, J.A. Laissue, A. Depaulis, R. Serduc, Scientific Reports June 2016
[101] "Absorbed dose-to-water protocol applied to synchrotron-generated X-rays at very high dose rates." Fournier, Pauline; Crosbie, Jeffrey; Cornelius, Iwan; Berkvens, P;Donzelli, Mattia; Clavel, Alexandre; Rosenfeld, Anatoly; Petasecca, Marco; Lerch, Michael; Bräuer-Krisch, Elke PMB-103491 2016
[102] Pauline Fournier, Cornelius, Iwan, Anatoly Rosenfeld, Elke Bräuer-Krisch, Petasecca, Marco; Lerch, Michael “X-Tream dosimetry of highly brilliant X-ray microbeams in the MRT hutch of the Australian Synchrotron”, Radiation Measurements 2017
[103] “Permeability of brain tumor vessels induced by uniform or spatially micro-fractionated synchrotron radiation therapies” Audrey Bouchet, Marine Potez, Nicolas Coquery, Claire Rome, Benjamin Lemasson, , Elke Bräuer-Krisch, Chantal Remy, Jean Albert Laissue, Emmanuel Barbier, Valentin Djonov, Raphaël Serduc. International Journal of Radiation Oncology, 1176-1182, 2017
[104] "Homogenous and microbeam X-ray radiation induces proteomic changes in the brains of irradiated rats and in the brains of non-irradiated cage mate rats" Richard Smith, Cristian Fernandez-Palomo, Jennifer Fazzar, Richard Smith, Elisabeth Schültke, Elke Bräuer-Krisch, Jean Laissue, Christian Schroll, Colin Seymour, Carmel Mothersill, Dose-Response 2017



