- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Structural biology
- Structure of a human excitatory neurotransmitter transporter
Structure of a human excitatory neurotransmitter transporter
Excitatory neurotransmission in the human brain requires fast removal of the transmitter glutamate from the synaptic cleft. Excitatory amino acid transporters perform this function and prevent cytotoxicity. Here, the first crystal structures of a human excitatory amino acid transporter have been solved. This has unravelled important molecular details of their function and pharmacology.
Over the last decade, the structural knowledge of human excitatory neurotransmitter transporters has been based on crystal structures of archaeal homologs that offered a first glimpse into their molecular mechanisms but fall short of explaining the functional and pharmacological complexity of human transporters. Using beamlines ID23-1, ID23-2, ID29 and ID30B, among others, the X-ray crystal structures of a thermostable human excitatory amino acid transporter (EAAT1cryst) have been solved.
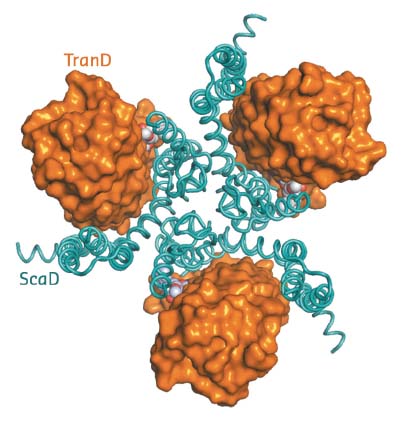
The structure of EAAT1cryst shows it to be a homotrimer (~180 kDa), in which each protomer contains two domains: a scaffold domain that forms all of the inter-subunit contacts, and a transport domain that contains all the residues to coordinate the substrate and coupled sodium ions (Figure 6). Previous structural work on homologs showed that substrate translocation across the membrane occurs through large rigid body movements of the transport domain relative to the scaffold domain with the substrate occluded in its core [1,2]. The EAAT1cryst structure captures a so-called outward-facing state of the transport cycle, in which the transport domain is in the ‘up-position’ with the neurotransmitter-binding site facing the extracellular side of the membrane.
 |
|
Fig. 6: Extracellular view of the EAAT1cryst trimer with the transport (TranD) and scaffold (ScaD) domains coloured orange and cyan respectively. |
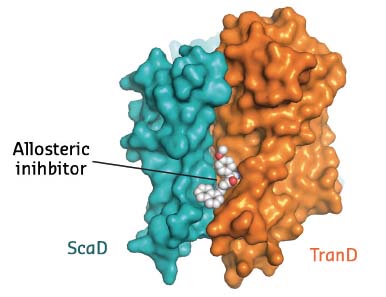
EAAT1cryst was crystallised in the presence of both the substrate and UCPH-101, the only known selective allosteric inhibitor of this family of proteins. Clear electron density showed the inhibitor wedged between the transport and scaffold domains of each protomer (Figure 7). In combination with studies of the transporter dynamics and functional assays, this crystallographic work thus unravelled the first allosteric mechanism of inhibition of excitatory neurotransmitter transporters: the allosteric compound ‘glues’ the transport and scaffold domains together and precludes the ‘elevator-like’ motions of the former that are required for transport.
 |
|
Fig. 7: Membrane view of a EAAT1cryst protomer with the allosteric compound UCPH-101 bound between the transport and scaffold domains. |
This work opens the way for a better understanding of the molecular mechanisms of the function, regulation and pharmacology of an essential component of the synaptic machinery in the brain.
Principal publication and authors
Structure and allosteric inhibition of excitatory amino acid transporter 1, J.C. Canul-Tec (a,b), R. Assal (a,b), E. Cirri (a,b), P. Legrand (c), S. Brier (b,d), J. Chamot-Rooke (b,d) and N. Reyes (a,b), Nature 544, 446-451 (2017); doi: 10.1038/nature22064.
(a) Molecular Mechanisms of Membrane Transport Laboratory, Institut Pasteur, Paris (France)
(b) UMR 3528, CNRS, Institut Pasteur, Paris (France)
(c) Synchrotron SOLEIL, Gif-sur-Yvette (France) (d) Structural Mass Spectrometry and Proteomics Unit, Institut Pasteur, Paris (France)
References
[1] N. Reyes et al., Nature 462, 880-885 (2009).
[2] N. Reyes et al., Nat. Struct. Mol. Biol. 20, 634-640 (2013).



