- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Structural biology
- Structural insights into a protein complex critical for cellular nutrient sensing
Structural insights into a protein complex critical for cellular nutrient sensing
A cooperative initiative of research groups from the Medical University Innsbruck have determined the crystal structure of a major cellular protein complex that has roles in sensing energy status and nutrient availability in cells and contributes to the activation of cell-growth controlling protein machinery.
The mechanistic target of rapamycin complex 1 (mTORC1) is a master regulatory protein complex for cellular growth, which responds to the availability of nutrients and associated cellular signals including energy status and other cues influenced by environmental factors [1]. The activity of the master regulator requires the mTORC1 complex to be recruited to the membrane of lysosomes, which are not only waste disposal systems but also mobile signalling platforms where availability of amino acids and other nutrient status determining compounds are sensed [2].
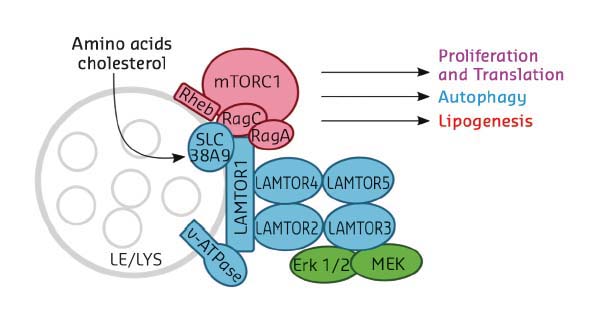
The master regulator LAMTOR (also termed Ragulator) is a pentameric late endosomal/lysosomal scaffold complex consisting of five protein components, LAMTOR1-5 (Figure 26), and serving as a point of convergence/integration of nutrient status and signalling. Lipid-modified LAMTOR1 anchors the remaining subunits, LAMTOR2-5, to the limiting membrane of the organelle. Rag GTPases are Ras-related switches that exist as heterodimers sharing active GTP-bound RagA/B and inactive GDP-bound RagC/D conformations. LAMTOR/Ragulator is important for tethering these Rag-GTPases to the lysosomal membrane to meet mTORC1, depending on availability of nutrients. Indeed, Ragulator has been reported to act as a guanine nucleotide exchange factor (GEF) activating Rags for interaction with mTORC1 [3].
 |
|
Fig. 26: Cartoon diagram showing the LAMTOR/Ragulator components in association with the late endosome/lysosome (LE/LYS) and with the mTORC1 complex. Selected cellular processes controlled by the complex are indicated. |
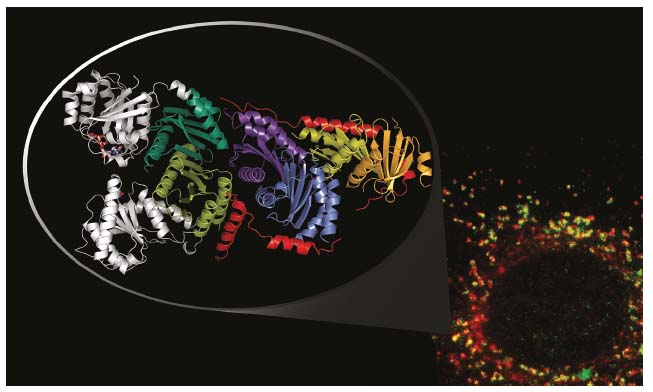
The structure of the pentameric Ragulator complex (LAMTOR1-5), determined from data collected at beamline ID23-2, reveals a heterotetramer composed of two heterodimers, LAMTOR2/3 and LAMTOR4/5, apparently held together by the LAMTOR1 component, of which the helix-rich core wraps around the four ‘roadblock’ components in a belt-like fashion (Figure 27). Cellular studies with CRISPR/Cas9 edited human cells demonstrated that loss of the interactions seen in the crystal structure led to complex destabilisation and impaired mTORC1 signalling upon amino acid derived stimulation after exposure.
Crystallisation of the heptameric Rag/Ragulator complex appeared to be challenging. However, as literature data pointed to a potential interface with the C-terminal dimerisation domains of the Rags, a complex between just those domains and Ragulator was purified and crystallised. The structure of this complex, derived from data collected at ID29, reveals a tight interaction between the C-terminal RagA/C roadblock dimer and the LAMTOR2/3 heterodimer. Alignment of the C-terminal roadblock domains with the crystal structure of the previously solved RagA/C homologue, Gtr1/Gtr2 (from yeast), immediately suggests a model for the full-length heptameric Rag/Ragulator complex (Figure 27). Most importantly, in this model the G-domains of the Rags, and thus their key nucleotide binding components (phosphate binding loop and switch regions), are exposed to the solvent rather than directly interacting with Ragulator components, as would have been expected for a canonical guanine nucleotide exchange factor.
 |
|
Fig. 27: Immunofluorescence image of LAMTOR components (red) and a lysosomal marker (LAMP1 in green, bottom right); co-localisation of both components is shown in yellow. The crystal structure of the truncated Rag/Ragulator complex is shown as a zoom inset (top left). Here, the belt-like LAMTOR1 component is shown in red, the G-domains (position derived from structural alignments) in grey. |
The structural model obtained above (Figure 27) has been recently confirmed by a hybrid study combining crystallography of the pentameric Ragulator with cryo-electron microscopy investigations of the heptameric complex containing RagA/C heterodimers. These latter also show the Rag G-domains in an exposed position and oriented to the solvent [4]. Additionally, three further crystallographic studies confirmed the crystal structure of the pentameric LAMTOR/Ragulator [5, 6, 7] and of the G-domain truncated heptameric Ragulator complex obtained above [4, 5, 6]. One of these studies revealed the lack of nucleotide exchange factor activity for the recombinant Ragulator complex and raises the possibility that previously reported activity could require one (or more) additional cellular components not present in recombinantly purified complex preparations [5]. Future studies will investigate this issue in greater detail.
The structural knowledge about the positions of critical amino acids positions revealed in these investigations provides a basis for the development of potential new drugs that could turn off the master regulatory complex in diseases like cancer or metabolic disorders in which the mTORC1 signalling pathway is overactive.
Principal publication and authors
Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling, M.E.G. de Araujo (a), A. Naschberger (b), B.G. Fürnrohr (b), T. Stasyk (a), T Dunzendorfer-Matt (b), S. Lechner (b), S. Welti (b), L. Kremser (c), G. Shivalingaiah (b), M. Offterdinger (d), H.H. Lindner (c), L.A. Huber (a) and K. Scheffzek (b), Science 358, 377 (2017); doi: 10.1126/science.aao1583.
(a) Division of Cell Biology, Biocenter, Medical University of Innsbruck (Austria)
(b) Division of Biological Chemistry, Biocenter, Medical University of Innsbruck (Austria)
(c) Division of Clinical Biochemistry, Biocenter, Medical University of Innsbruck (Austria)
(d) Division of Neurobiochemistry-Biooptics, Biocenter, Medical University of Innsbruck (Austria)
(e) Austrian Drug Screening Institute, Innsbruck (Austria)
References
[1] D.M. Sabatini, PNAS 114, 11818 (2017).
[2] M. Rebsamen et al., Nature 519, 477 (2015).
[3] L. Bar-Peled et al., Cell 150, 1196 (2012).
[4] M.Y. Su et al., Mol Cell 68, 835 (2017).
[5] T. Zhang et al., Nat Commun 8, 1394 (2017).
[6] R. Yonehara et al., Nat Commun 8, 1625 (2017).
[7] Z. Mu et al., Cell Discovery 3, 17049 (2017).



