New technique for fast chemical contrast: X-ray fluorescence intensity ratio detection at ID26
A new protocol has been created for efficient detection of the chemical state of an element based on X-ray emission (fluorescence) spectroscopy using a Bragg optics spectrometer. Differences between chemical states in spatially heterogeneous or temporally evolving samples can be identified in favourable cases within a timescale of milliseconds.
Fast element-selective characterisation of the chemical state of the ions in a sample is desirable in studies across many fields of natural science. Possible applications include screening of a large number of samples, spatial mapping and imaging or time-resolved experiments. Fast-scanning X-ray monochromators were developed at synchrotron radiation sources to measure X-ray absorption spectroscopy (XAS) in transmission mode with frequencies up to 200 Hz to follow chemical reactions. However, many studies require XAS detection in X-ray fluorescence mode because of low analyte concentration or the experimental conditions around the sample (e.g., in-situ studies). The data acquisition rate is limited in X-ray fluorescence-detected XAS using energy-resolving solid-state detectors by the non-linearity of the photon detector at high photon fluxes. This may prevent sufficient quality data at sub-second rate.
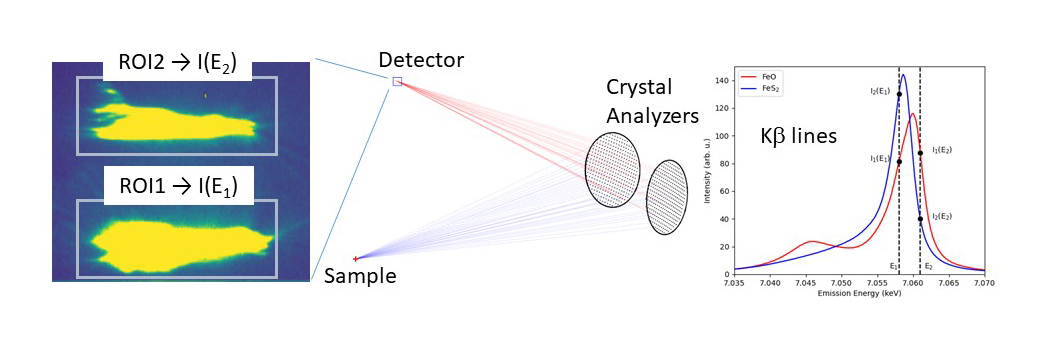
X-ray emission (or X-ray fluorescence) also shows a chemical sensitivity (e.g., Fe Kβ lines in Figure 1). At beamline ID26, a new protocol has been developed by simultaneously recording the X-ray fluorescence intensity I(E) at two appropriately chosen emission energies. For a multi-crystal scanning spectrometer, this is efficiently achieved by tuning some of the crystals to one energy and the remaining crystals to another energy (Figure 1). The ratio of fluorescence intensities that are separated on a pixel detector and recorded at two well-chosen fluorescence energies does not need to be normalised to the incoming photon flux. The incident energy can be chosen freely above the absorption edge of the analyte atom.
Click image to enlarge
Fig. 1: Experimental setup. At least two (possibly more) crystal analysers are tuned to two different X-ray fluorescence energies that are separated on a pixel detector (left panel). The Kβ lines in two Fe compounds are shown in the right panel to illustrate the chemical dependence. ROI: region of interest. A possible choice of the two X-ray fluorescence energies is indicated by the vertical bars.
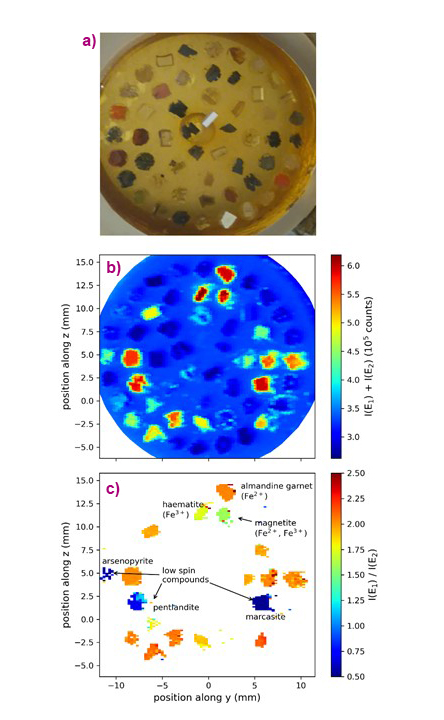
The new protocol was applied to a set of mineral standards that contained Fe in different concentrations and chemical states. Figure 2 shows the different information obtained. The sum of the intensities at the different emission energies gives the elemental map, which can also be obtained using an energy-resolving X-ray detector. The spectral shape of the Kβ lines changes with any modification of the electron spin density around Fe, which may be induced by changes of the oxidation or spin state but also by any change in the covalent bond that affects the 3d orbitals. The ratio of the two intensities thus provides a map of the chemical state of Fe. The map shows that subtle changes, such as between Fe3O4 and Fe2O3, can also be detected. The strongest spectral contrast is observed between Fe-containing minerals in high-spin or low-spin electronic states.
Click image to enlarge
Fig. 2: a) Photo of the mineral standards. b-c) X-ray maps of the standards using the Fe Kβ fluorescence: (b) Fluorescence intensity giving an element (Fe) map and (c) ratio of intensities showing the chemical state of Fe in the samples. The contrast was recorded for the energy pair (E1, E2) = (7057.7, 7060.1) eV.
Over dispersive X-ray emission that simultaneously records a full spectrum, the advantage of this new protocol, named X-ray fluorescence intensity ratio detection (XFIRD), is two orders of magnitude higher detection efficiency (defined as the required acquisition time to record a spectral difference). This gain arises from the more efficient use of the available crystal analyser surface. Consequently, a lower X-ray dose is delivered to the sample. This gain in detection efficiency comes with a loss in spectral information. However, the information that is discarded by not recording a full spectrum may not fundamentally change the scientific findings.
Depending on the analyte concentration and the spectral contrast among the targeted species, acquisition times of a few milliseconds may suffice to characterise the chemical state. It is planned to use this protocol for spatial mapping of reactors in catalysis and in electrochemical cells and to follow reaction kinetics. Combination of XFIRD with XRD would allow one to obtain atomic and electronic structural information simultaneously.
Principal publication and authors
Fast Chemical Contrast by X-ray Fluorescence Intensity Ratio Detection, B. Detlefs, S. Graziano, P. Glatzel, Anal. Chem. 95, 23, 8758-8762 (2023); https://pubs.acs.org/doi/10.1021/acs.analchem.3c00623
ESRF