- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- X-ray nanoprobe
- Nucleation and growth of CuInS2 nanocrystals studied in situ using synchrotron X-ray diffraction
Nucleation and growth of CuInS2 nanocrystals studied in situ using synchrotron X-ray diffraction
Synchrotron X-ray diffraction is used to monitor in situ the formation of CuInS2 nanocrystals. SAXS measurements show that the reaction intermediate formed at 100°C presents a periodic lamellar structure. WAXS measurements of the CuInS2 nanocrystals at 230°C demonstrate that their growth kinetics depend on the degree of precursor conversion achieved at 100°C.
Ternary metal chalcogenide nanocrystals (NCs) are explored for various types of applications in the fields of energy conversion (solar cells, photocatalysis, thermoelectrics), light emission and biological detection [1, 2]. Their optical and electronic properties can be tuned by changing the NC size (quantum confinement effect) or by varying the composition. Within this family, CuInS2 (CIS) NCs are the most widely studied due to their outstanding luminescence properties and their well-established chemical synthesis. It involves mixing the metal precursors (copper iodide and indium acetate) with dodecanethiol (DDT) and heating first to 100°C (30-60 min) for complexation of the precursors and then to 230°C (15-60 min) for the growth of the NCs. Despite the simplicity of this procedure, the reaction mechanism and surface state of the obtained NCs remain elusive; they are the focus of the present study.
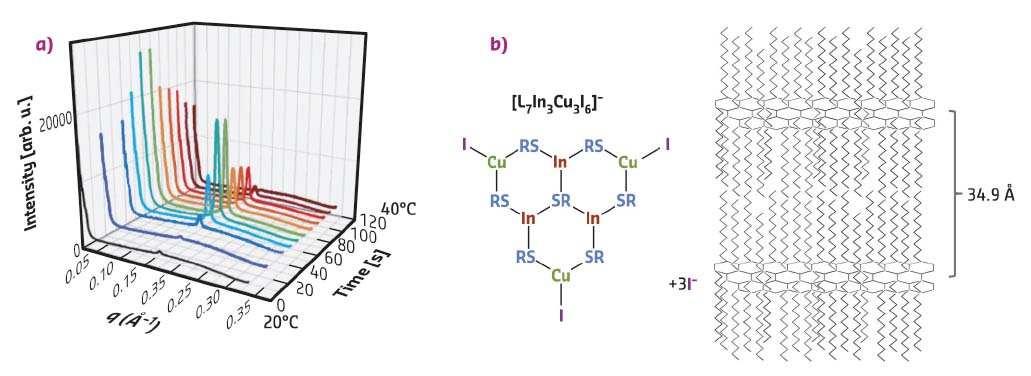
With the goal to monitor in situ the nucleation and growth stages of CIS NCs, the synthesis reaction was carried out inside a thin glass capillary. It contained the reaction intermediate obtained after heating the precursors at 100°C and was placed into a heating stage mounted on the sample goniometer at beamline ID01 set at λ = 0.774 Å. A large q range was accessible by varying the distance of the 2D detector. The capillary was heated from room temperature to 230°C while performing SAXS and WAXS measurements. The data recorded at low temperatures (20-40°C) exhibit a distinct peak at q = 0.18 Å-1 (Figure 86a). As confirmed by ex situ SAXS measurements, this peak originates from a periodic lamellar structure with a characteristic distance of 34.9 Å, corresponding to twice the length of a DDT ligand. Additional ESI-mass spectrometry enables the hypothetical structure of the reaction intermediate to be drawn (shown in Figure 86b), in which metal and µ3-bridging S atoms form a hexagonal network and alkyl chains are distributed on both sides of this slab.
 |
|
Fig. 86: a) Synchrotron in situ SAXS measurements performed during the heating of the CIS precursor solution in a glass capillary from 20°C to 40°C within 2 min (preheating time at 100°C: 60 min). b) Largest fragment of the reaction intermediate detected by ESI-mass spectrometry (L = SC12H25–) and visualisation of the expected lamellar structure formed after heating the precursors to 100°C and cooling to room temperature. |
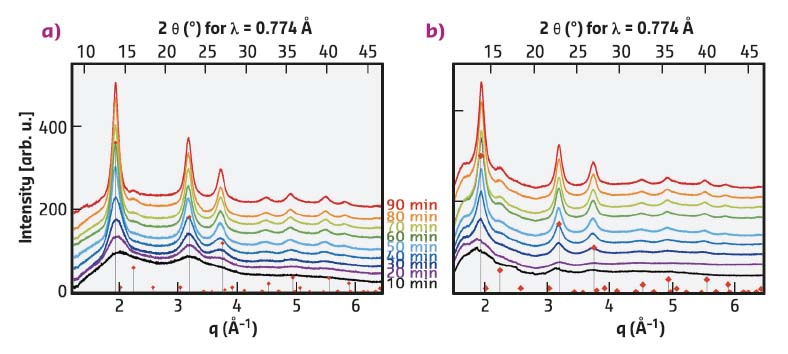
During temperature increase, the SAXS peak, and hence the lamellar ordering, disappears. At around 180°C, nucleation of the CIS NCs starts but WAXS measurements do not yield any exploitable signals for heating times < 10 min at 230°C. The diffractograms obtained for longer times (Figure 87) correspond to CIS in the tetragonal chalcopyrite or cubic zinc blende structures, which cannot be distinguished here. Closer analysis of the peak line width using the Scherrer formula reveals that the growth kinetics of the NCs are strongly correlated with the preheating time of the precursors at 100°C: sample A (preheating time: 30 min) grows much faster and also has a slightly larger final particle size (5.0 nm) than sample B (preheating time: 60 min, 4.2 nm). While optically the two precursor solutions do not exhibit any difference, sample A does not show the characteristic SAXS peak seen in Figure 86a.
 |
|
Fig. 87: In situ synchrotron WAXS measurements at 230°C, as a function of the preheating time at 100°C (red diamonds: diffraction pattern of chalcopyrite CIS, JCPDS: 04-002-6388). Preheating time of the precursor solution at 100°C: a) 30 min, b) 60 min. |
An explanation for the different behaviours is as follows: In sample B the complexation of the metal precursors by thiolate molecules is accomplished. This favours cleavage of the S-C bond in dodecanethiolate, which is the only sulfur source in the system, and initially generates a large number of nuclei. In sample A the precursor conversion is not complete due to the shorter heating at 100°C. Therefore a lower number of nuclei are initially formed, which then grow to larger final particles. Beside the inorganic core, the nature, binding mode and surface density of the organic ligands capping the obtained CIS NCs are also studied using 1D and 2D proton and carbon NMR spectroscopy.
Principal publication and authors
Growth Mechanism and Surface State of CuInS2 Nanocrystals Synthesized with Dodecanethiol, M. Gromova (a), A. Lefrançois (b), L. Vaure (b), F. Agnese (a), D. Aldakov (b), A. Maurice (b), D. Djurado (b), C. Lebrun (2), A. de Geyer (a), T.U. Schülli (c), S. Pouget (a) and P. Reiss (b), J. Am. Chem. Soc. 139, 15748-15759 (2017); doi: 10.1021/jacs.7b07401.
(a) Univ. Grenoble Alpes, CEA, INAC, MEM, Grenoble (France)
(b) Univ. Grenoble Alpes, CEA, CNRS, INAC, UMR5819 SyMMES, Grenoble (France)
(c) ESRF
References
[1] D. Aldakov et al., J. Mater. Chem. C. 1, 3756-3776 (2013).
[2] M. Sandroni et al., ACS Energy Lett. 2, 1076-1088 (2017).



