- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Structural biology
- First atomic-resolution snapshots of egg coat-sperm interaction at fertilisation
First atomic-resolution snapshots of egg coat-sperm interaction at fertilisation
Crystallographic studies of egg coat components VERL and ZP2 show that a conserved protein fold mediates interaction with sperm in both invertebrates and vertebrates. Structures of VERL bound to lysin, its counterpart molecule on sperm, reveal the molecular basis of species-restricted gamete recognition, leading to a model of egg coat architecture and penetration by sperm.
By transmitting the genetic information to the next generation and marking the beginning of a new individual, the encounter between gametes at fertilisation constitutes one of the most fundamental processes of life. Although this crucial event has been studied for centuries, the molecular basis of how sperm recognises the extracellular coat of the egg at the onset of fertilisation remained unknown. Similarly, it was unclear how sperm subsequently penetrates the egg coat – called zona pellucida (ZP) in mammals and vitelline envelope (VE) in non-mammals – in order to fuse with the plasma membrane of the egg. Based on previous studies of the major ZP component ZP3 [1], it was proposed that a common ‘ZP-N’ fold is adopted by the domain repeats of ZP2 and VERL, two sperm-interacting subunits of the mammalian ZP and mollusk VE, respectively. This suggestion had important functional implications because, whereas a binding partner of ZP2 remains to be identified, VERL is known to interact with lysin, an amphipathic sperm molecule that dissolves the VE non-enzymatically [2]. However, as amino acid sequence identity between ZP2 and VERL repeats is a mere 10%, this hypothesis remained highly speculative.
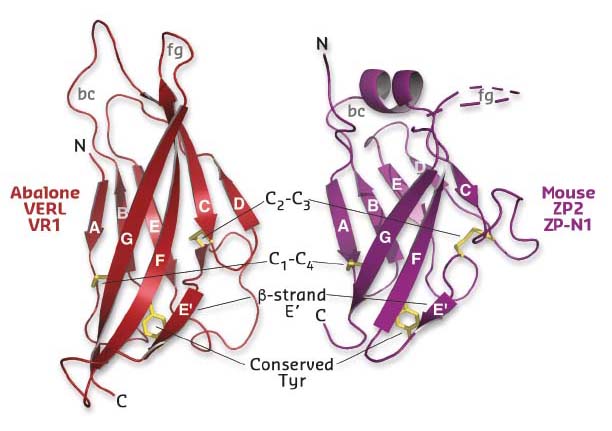
Using diffraction data collected at beamlines ID29 and ID23-2, a series of crystal structures of the first three repeats of red abalone VERL (VR1-3) and the N-terminal ZP-N1 domain of mouse ZP2 was determined (Figure 22). Comparison of these conclusively showed that, despite 600 million years of evolutionary divergence, mollusk and mammalian egg coat proteins use the same basic architecture to interact with sperm.
 |
|
Fig. 22: Structural comparison of mollusk (left) and mammalian (right) egg coat proteins shows that their repeats share a common ZP-N domain fold, whose characteristic features are indicated. |
Whereas lysin and the first two repeats of VERL evolve rapidly under positive Darwinian selection, the remaining twenty repeats are homogenised by concerted evolution. To understand how this evolutionary pattern affects gamete recognition, biochemical assays of VERL-repeat-lysin interactions were performed and quantified by microscale thermophoresis. Together, these experiments showed that lysin does not bind highly sequence-divergent VR1, interacts weakly, but species-specifically, with moderately sequence-divergent VR2 (Kd ~500 nM) and forms a tight non-species-specific complex with conserved VR3 (Kd ~2 nM). Mutational analysis suggested that divergence of the N-terminal sequence of VERL inactivated VR1 and lowered the binding affinity of VR2 compared to VR3-22, thus generating species-specificity by amplifying the effect of positive selection on lysin.
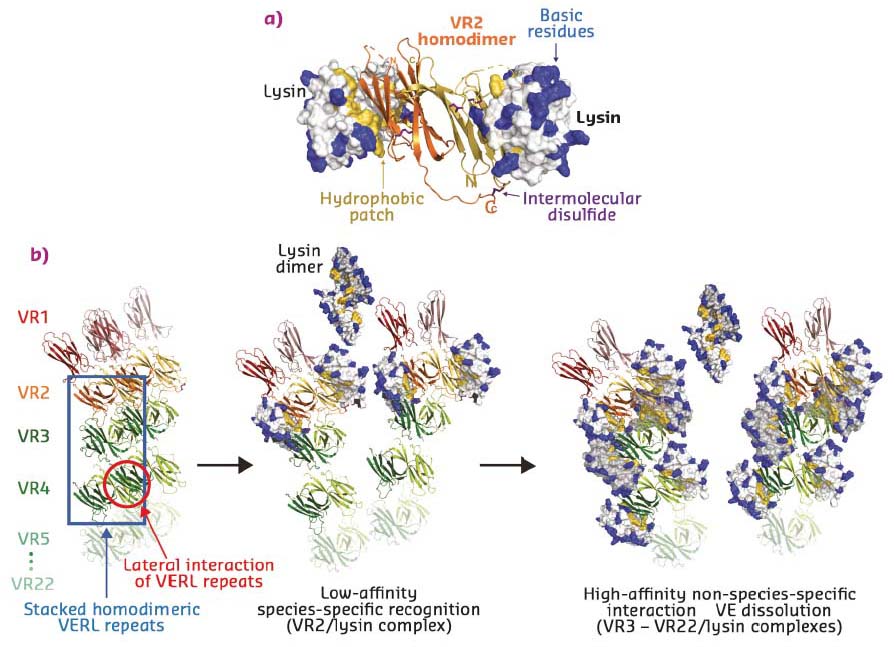
To determine the molecular basis of egg coat-sperm interaction, VR2-lysin and VR3-lysin complexes were crystallised and their structures solved using datasets collected at ID29 and ID23-1. VR2, which contains two additional Cys residues compared to other VERL repeats, forms an antiparallel homodimer, containing an intermolecular disulfide-bond between monomers. These hydrophobically bind two copies of lysin on the opposite faces of the dimer (Figure 23a). VR3 forms a similar 1:1 complex with lysin, but – consistent with a higher affinity interaction – this involves a larger number of contacts.
Taken together with VERL-VERL contacts observed in the respective crystals, the structures suggest a model whereby two intertwined VERL molecules generate a filament branch that exposes VR1 repeats on the surface of the VE (Figure 23b, left panel). These are followed by the covalently bound antiparallel VR2 homodimer, whose low affinity for lysin acts as a species-specific checkpoint for sperm attachment. In the absence of lysin, adjacent VERL branches are held together by interaction between their hydrophobic patches. Upon deposition of weak lysin dimers onto the VE, the sperm protein’s hydrophobic interface replaces the lateral interactions between VERL branches. This results in binding of monomeric lysin to the egg coat and juxtaposition of many copies of its highly positively charged surface on the repeats of adjacent VERL branches. As supported by molecular dynamics simulations, the ensuing electrostatic repulsion then pushes the VERL branches apart and disrupts the egg coat, ultimately generating a hole for sperm penetration and fusion (Figure 23b, middle and right panels).
 |
|
Fig. 23: a) Structure of the VERL VR2-sperm lysin complex. The VR2 homodimer is shown in cartoon representation and lysin is depicted as surface with basic and hydrophobic residues coloured blue and yellow, respectively. b) Model of higher-order VERL organisation and its unravelling by lysin at fertilisation. |
In summary, this study provides unprecedented insights into the molecular basis of the very first step of fertilisation. At the same time, considering that ZP2 ZP-N1 contains a region thought to play a crucial role in human gamete recognition [3], the findings have important implications for reproductive medicine.
Principal publication and authors
Structural basis of egg coat-sperm recognition at fertilization, I. Raj (a,c), H. Sadat Al Hosseini (a,c), E. Dioguardi (a), K. Nishimura (a), L. Han (a), A. Villa (a), D. de Sanctis (b) and L. Jovine (a), Cell 169, 1315-1326 (2017); doi: 10.1016/j.cell.2017.05.033.
(a) Department of Biosciences and Nutrition & Center for Innovative Medicine, Karolinska Institutet, Huddinge (Sweden)
(b) ESRF
(c) These authors contributed equally
References
[1] M. Monné et al., Nature 456, 653-657 (2008).
[2] C.A. Lewis et al., Dev. Biol. 92, 227-239 (1982).
[3] M.A. Avella et al., J. Cell. Biol. 205, 801-809 (2014).



