- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Electronic structure, magnetism and dynamics
- Thallium: a hidden hazard within drinking water pipelines
Thallium: a hidden hazard within drinking water pipelines
Micrometre-sized aggregates of Tl2O3 and TlCl have been detected in rust scales in drinking water pipelines, using X-ray diffraction, scanning electron microscopy, and X-ray absorption spectroscopy. A possible mechanism is suggested for the formation of secondary sources of Tl contamination in the pipelines due to chlorine-based oxidants added at water treatment plants.
Thallium (Tl) is one of the most toxic elements for living organisms. In Pietrasanta, a small town in northern Tuscany, Italy, mineralogical and geochemical studies [1] had pointed out the occurrence of high Tl contents (up to 1100 μg/g) in the pyrite ores that had been exploited in the area for several decades up to the end of the 1980s. This finding emphasised the threat related to the dispersion of Tl in the environment following pyrite oxidation.
In September 2014, Tl contents up to 10 μg/L were detected in the drinking water tapped from public fountains. Once the contaminated spring had been located, it was replaced by another spring and its water, having a low Tl content (less than 0.10 μg/L), was introduced in the drinking water distribution system. Unexpectedly, the Tl content in water increased up to 80 μg/L. A new source of Tl was at the origin of the contamination and it was hypothesised that this could be due to pipeline encrustations.
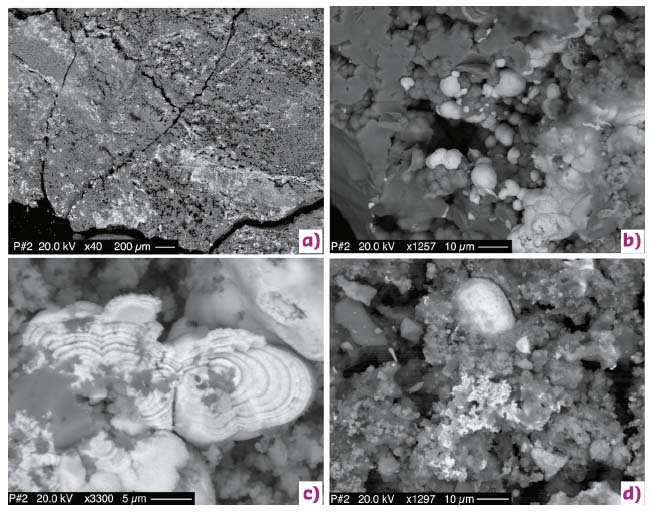
Following their removal from the water distribution system, four segments of the pipeline were made available for study. Their interior was heterogeneous, with centimetre-sized patchy areas from light orange to black in colour. X-ray fluorescence (XRF) spectroscopy showed that a striking difference in the Tl content of the rust scales lining the pipeline interior occurred as a function of their position with respect to the water treatment plant: before the plant the rust scales had a low Tl content, whereas after the plant (e.g., samples P#2 and P#3) Tl contents up to 5.3 wt% were measured. The difference was related to the occurrence, in the latter sample, of micrometre-sized globular aggregates of trivalent Tl(III) thallium oxide Tl2O3 (Figure 109). The rust scales were peppered with these small aggregates, as confirmed both by scanning electron microscopy observations, coupled with EDS chemical analysis, and X-ray powder diffraction.
 |
|
Fig. 109: Back-scattered electron images of rust scales showing details of the highly reflective, Tl-rich phases. a) Small fragment of sample P#2 showing highly reflective spots peppering the surface; b) micrometre-sized spherules of Tl2O3 (light grey) on Fe-hydroxides (dark grey); c) micrometre-sized spherules of Tl2O3 (light grey) showing their onion-shell internal structure; d) Tl2O3 microspherule (light grey, upper centre) close to a tiny TlCl encrustation (light grey, lower centre). |
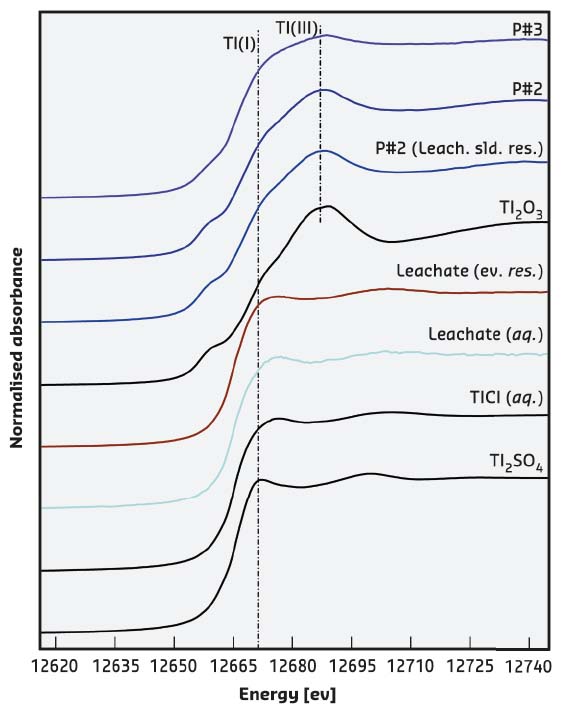
However, since the solubility of Tl2O3 in water is known to be very low, it was unlikely that the high Tl concentrations in the drinking water were related to the occurrence of this Tl salt within the pipeline. In addition, leaching and drying experiments on the rust scales revealed that the precipitated salt was monovalent Tl(I) thallium chloride TlCl, a more soluble salt. The data suggested the occurrence of another source of contamination so X-ray absorption spectroscopy (XAS) measurements were taken at the CRG beamline LISA-BM08. Figure 110 shows the XANES spectra measured for samples and standards. They confirmed not only the occurrence of Tl(III), related to the presence of Tl2O3, but demonstrated also the occurrence of a phase containing Tl(I), likely coordinated by Cl. Moreover, the XAS data collected on the leachate samples clearly supported the presence of a soluble Tl(I) compound. This finding promoted a further SEM investigation and finally micrometre-sized aggregates of nanometre-sized crystals of TlCl were identified (Figure 109).
 |
|
Fig. 110: Tl L3-edge XANES of measured samples and model compounds. Main edge crests (dashed line) are found at ~12681 and ~12687 eV for Tl(I) and Tl(III) compounds, respectively. Samples from rust scales (from P#2 to P#3 with increasing distance from the treatment plant) are congruous with those of Tl2O3; the presence of a shoulder in correspondence of the Tl(I) edge position suggests the presence of two different signals from both Tl(I) and Tl(III). On the other hand, the leachate samples show the presence of the sole Tl(I). |
The study suggests that the Tl dissolved as Tl(I) in the water from the contaminated spring was removed when the oxidants NaOCl/ClO2 were added during the water treatment, through oxidative precipitation of Tl2O3 and precipitation of minor TlCl. This process has a two-fold significance: it decreases the bioavailability of this element due to the precipitation of its compounds, but it favours the deposition of Tl-rich coatings within the pipelines, giving rise to a secondary and unexpected source of contamination.
Thallium is a relatively rare but widely dispersed element, and owing to its difficult detection using classical analytical methods, it is not unlikely that its passage from lithosphere to hydrosphere could be more common than thought. In addition, the thallium-rich nature of the rust scales is the result of processes integrated over a period of several years. Consequently, the presence of even low Tl concentrations could represent a hidden hazard seriously affecting the quality of drinkable water.
Principal publication and authors
Thallium-rich rust scales in drinkable water distribution systems: A case study from northern Tuscany, Italy, C. Biagioni (a), M. D’Orazio (a), G.O. Lepore (b), F. d’Acapito (b) and S. Vezzoni (a), Sci Total Environ. 587-588, 491-501 (2017); doi: 10.1016/j.scitotenv.2017.02.177.
(a) Dipartimento di Scienze della Terra, Università di Pisa (Italy)
(b) CNR-IOM-OGG c/o ESRF-LISA CRG, Grenoble (France)
References
[1] M. D’Orazio et al., Mineral. Dep. 52, 687 (2017).



