- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Electronic structure, magnetism and dynamics
- Chemical reaction of cinnamic acid followed by time-resolved X-ray Raman spectroscopy
Chemical reaction of cinnamic acid followed by time-resolved X-ray Raman spectroscopy
Bonding between atoms to form molecules originates from the electronic structure of the system, which changes in chemical reactions. In this spectroscopic study, the changes in electronic structure were monitored in real time on a timescale of minutes for a crystal undergoing a dimerisation reaction. The study yields information on the reaction progression and demonstrates the ability of a novel imaging technique to map the chemical composition inside the macroscopic solid-state sample.
In crystalline materials, ultraviolet light can be used to induce topochemical reactions, in which pathways and products are governed by the crystal structure of the material. This offers an opportunity to synthesis processes with an amazing specificity and removes the need for solvents allowing more environment-friendly processes [1]. However, the dependence of the reaction pathways on photon energy is poorly understood, especially in the energy range of X-rays, where core-excited molecular species are created. Thus, novel X-ray techniques for probing these reactions are desirable as they can yield new results by allowing access to novel reactions in a variety of thermodynamic conditions.
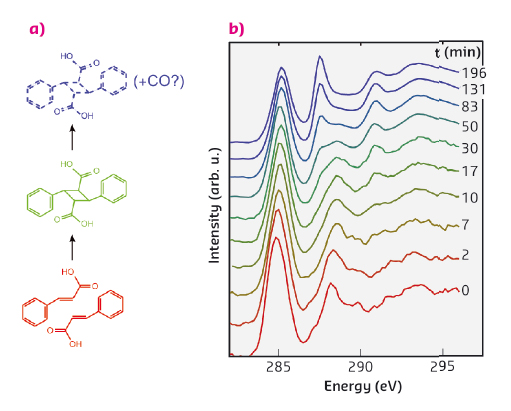
A canonical example of a topochemical reaction is the dimerisation of crystalline cinnamic acid. In this reaction, depicted in Figure 65a, two cinnamic acid molecules are bound together to form a dimer, a truxillic acid molecule. Previously it has been shown that the kinetics of this transformation, when induced by UV illumination, can be described by a Johnson-Mehl-Avrami-Kolmogorov (JMAK) model. The JMAK model describes reactions proceeding via formation of nuclei and their subsequent growth. In the current case the growth of the nuclei can be understood by noting that the dimerisation can occur preferentially close to the existing dimers.
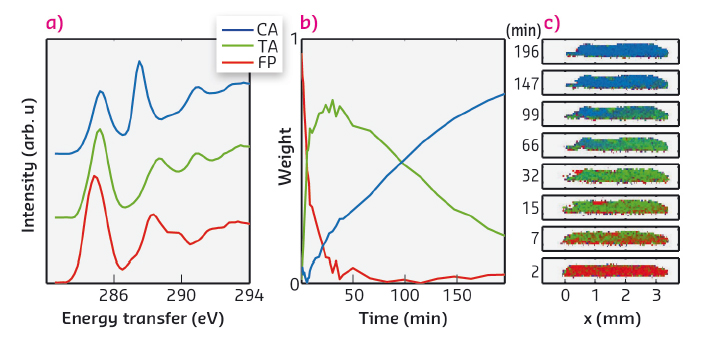
In our time-resolved in situ study, performed at beamline ID20, we observed this reaction to be induced by X-rays for the first time. The carbon K-edge spectra (Figure 65b) measured using non-resonant X-ray Raman spectroscopy (XRS) show on a short timescale the effect of dimerisation, which we explain in detail with the help of spectral simulations. The dimerisation competes with another reaction that occurs at a slower rate and seems to produce carbon monoxide. Spectral decomposition using nonnegative matrix factorisation allowed us to obtain the component spectra and time-dependent weights for cinnamic acid, truxillic acid, and final products (Figure 66a and b). To quantify the reaction kinetics, we fit a JMAK-reaction model to the component weights, the results of which suggest that the X-ray induced dimerisation proceeds more homogeneously than the UV-induced reaction.
 |
|
Fig. 65: (a) The reaction scheme of the dimerisation of α-trans-cinnamic acid to α-truxillic acid and a subsequent disintegration reaction. (b) The time-resolved carbon K-edge spectra show the effects of dimerisation on a short time-scale (0-17 minutes) and disintegration on a longer time-scale. The spectra are offset for clarity. |
We also utilised the ability of ID20 for direct tomography with spectral contrast [2]. In this imaging technique chemical bonding inside the sample is mapped using the XRS spectrum. The incident X-ray beam illuminates a section of a sample, from which the scattered X-rays are recorded to form a hyperspectral image that contains an individual spectrum for each voxel. We utilised this method for following the chemical composition within the crystal. Figure 66c shows the images of a section of the crystal as a function of time, with colours representing the weights of each component spectrum (Figure 66a) fitted to the hyperspectral images. The reaction rate is seen to be higher in the front of the crystal, where the dose rate is higher, than at the back. Our work paves the way to other novel time-resolved in situ studies of X-ray-induced chemical reactions using XRS for spectral imaging.
 |
|
Fig. 66: (a) The component spectra of α-trans-cinnamic acid, α-truxillic acid, and final products (CA, TA, and FP, respectively). (b) The weight of each component as a function of irradiation time. (c) The progression of the chemical composition inside the sample crystal mapped by hyperspectral imaging. Red, green, and blue channel in each pixel corresponds to the weight of CA, TA, and FP components, respectively. |
Principal publication and authors
X-ray induced dimerization of cinnamic acid: time-resolved inelastic X-ray scattering study, J. Inkinen (a), J. Niskanen (a), T. Talka (a), C.J. Sahle (b), H. Müller (b), L. Khriachtchev (c), J. Hashemi (a), A. Akbari (a), M. Hakala (a) and S. Huotari (a), Scientific Reports 5, 15851 (2015); doi: 10.1038/srep15851.
(a) Department of Physics, University of Helsinki (Finland)
(b) ESRF
(c) Department of Chemistry, University of Helsinki (Finland)
References
[1] K. Biradha and R. Santra, Chem. Soc. Rev. 42, 950–967 (2013).
[2] S. Huotari et al., Nat. Mater. 10, 489–493 (2011).



