- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- X-ray Imaging
- How can local environmental conditions influence the secondary structure of an amyloid beta peptide?
How can local environmental conditions influence the secondary structure of an amyloid beta peptide?
Extracellular amyloid plaques are a hallmark material for the study of Alzheimer’s disease. The neurotoxicity of their main components, the amyloid b peptides (Aβs), may be mediated by their direct interaction with the neural lipid membrane. In order to shed more light on conformational and structural processes of amyloidosis involving membranes, we focused our attention on the Aβ(25−35) and Aβ(1-42) species in the presence of a phospholipid mixture that mimics the phospholipid composition of the neural membranes, i.e., a lipid mixture made of POPC (1-palmitoyl-2-oleoylphospatidylcholine) and POPS (1-palmitoyl-2-oleoylphospatidylserine).
 |
|
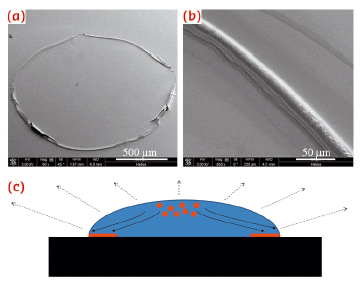
Fig. 70: a,b) SEM micrographs of Aβ(25−35) ring-like residues; c) Sketch of an evaporating droplet on a hydrophilic substrate. The evaporation rate is stronger near the contact line, provoking a radial convective motion of the solute from the centre toward the contact line. |
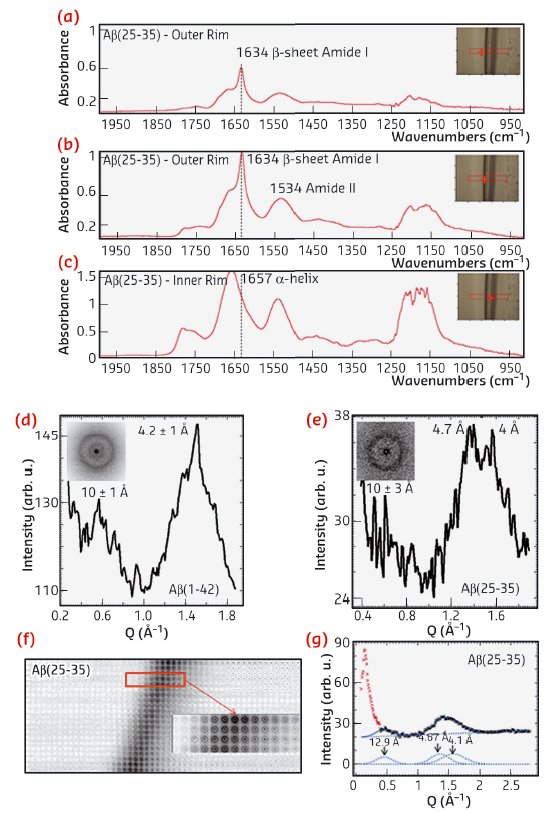
The investigation was supported by a multi-technique approach based on a combination of micro-Raman (μRaman), synchrotron radiation (SR) micro-Fourier-transform infrared spectroscopy (μFTIR) at ID21 and micro X-ray diffraction (μXRD) at ID13. In order to enhance the sensitivity of these probes, we deposited droplets with Aβ and phospholipid suspensions on highly hydrophilic nanostructured substrates. Ring-like solid residues were formed almost immediately due to the strong heterogeneous evaporation rate on hydrophilic substrates (Figure 70a,b). The aid of micro-fabricated supports for structural studies had already been successfully exploited in previous experiments [1]. As detected by µFTIR, the outer rim of Aβ-25-35 residue (Figure 71a,b) was characterised by β-sheet material (peak at 1634 cm-1), while the inner zone (Figure 71c) contained peptide in α-helical conformation (1657 cm-1). The high evaporation flux at the triple contact line resulted in an accumulation of solute while the inner regions were depleted (Figure 70c). Therefore, the convective flow and the enhanced peptide concentration in proximity to the external rim, have been shown to result in self-assembly of β-amyloid fibrils. At the inner rim, the lower amount of material was not sufficient for the α/β transition and the peptide remained arrested in a α-helical conformation. The Aβ(25−35)/POPC/POPS mixture showed, on the contrary, the exclusive presence of β-sheet material without any trace of α-helices indicating that the presence of phospholipids plays an active role in the fibrillation process, as already suggested by previous studies [2]. As a control, μFTIR experiments on Aβ(1−42) in the presence and absence of POPC/POPS revealed in both cases the presence of parallel β-sheet material. Complementary information on the crystalline fractions of the lipid and peptide system was obtained by micro small and wide angle X-ray scattering (μSAXS/WAXS). The pure Aβ(1−42) pattern (Figure 71d) showed β-sheet features with a 4.2 Å peak characteristic of the distance between hydrogen-bonded strands and a 10 Å peak due to the β-sheet stacking. In contrast, the Aβ(25−35) rim showed only a very weak signature of a peak in the range of 10 Å, suggesting stacking disorder (Figure 71e) and was further confirmed by a more detailed analysis of the peak profile by fitting Gaussian functions (Figure 71g). A composite peak with d ~4.7/4.1 Å (Figure 71g) agreed with the possible coexistence of a β-phase (4.1 Å) together with an α-phase (~4.7 Å).
 |
|
Fig. 71: a,b,c) FTIR spectra recorded from several regions of the Aβ(25−35) residue dried on highly hydrophilic BaF2 surfaces. Typical μXRD mesh scan and relative azimuthal averages of the Aβ(1−42) (D)and Aβ(25−35) (e) droplet rims. (f) XRD mesh-scan of the Aβ(25−35) solid rim. (g) Peak fitting of the Aβ(25−35) XRD pattern of Figure 71e. |
Based on these results, we were able to demonstrate the presence of an α/β transition from the internal rim of the coffee-ring residues to the external rim in the pure Aβ(25−35) case, probably due to the convective flow inside the droplet sitting on highly hydrophilic substrates that enhances the local concentration of the protein at the external edge of the drying drop. In contrast, the presence of POPC/POPS lipids in the protein did not result in α-helical structures and introduced the presence of pure β-type material. An integrative μXRD analysis of the same residues confirmed the μFTIR experiments. Further work could help to clarify the role of enzymes, such as acetylcholinesterase, and of different lipid systems in the amyloidosis of the analysed peptides and of other Aβ fragments.
Principal publication and authors
A. Accardo (a), V. Shalabaeva (a), M. Cotte (b), M. Burghammer (b), R. Krahne (a), C. Riekel (b) and S. Dante (a), Langmuir 30, 3191−3198 (2014).
(a) Istituto Italiano di Tecnologia, Genova (Italy)
(b) ESRF
References
[1] A. Accardo, E. Di Fabrizio, T. Limongi, G. Marinaro and C. Riekel, Journal of Synchrotron Radiation 21, 643-653 (2014).
[2] A.M. Sheikh and A. Nagai, FEBS Journal 278, 634−642 (2011).



