- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Dynamics and extreme conditions
- Structural transition in metallic dense oxygen
Structural transition in metallic dense oxygen
Solid oxygen exhibits both simple molecular and magnetic properties. Below 10 GPa, dense solid oxygen is a spin-controlled crystal and as such exists under four structural arrangements presenting different magnetic ordering. Above 10 GPa, solid oxygen transforms into a dark-red solid known as the ![]() phase. The exact structure of the
phase. The exact structure of the ![]() phase (monoclinic, C2/m) was determined only recently, revealing an intriguing condensed state for which the association of four O2 molecules into O8 entities prevail [1,2]. Beyond the stability field of its
phase (monoclinic, C2/m) was determined only recently, revealing an intriguing condensed state for which the association of four O2 molecules into O8 entities prevail [1,2]. Beyond the stability field of its ![]() phase, i.e., above 96 GPa, solid oxygen has been shown to present a metallic state corresponding to the molecular
phase, i.e., above 96 GPa, solid oxygen has been shown to present a metallic state corresponding to the molecular ![]() phase. The study of the metallic
phase. The study of the metallic ![]() phase has remained an outstanding experimental challenge for years because of the weak scattering power of oxygen in the 100 GPa range. Characterisation of the structural changes at the onset of metallisation is essential to understand the mechanism of the insulator-to-metal transition. Advanced single crystal growth techniques at high pressure and temperature and use of optimised conditions for performing microdiffraction are key to unravelling the elusive nature of metallic oxygen.
phase has remained an outstanding experimental challenge for years because of the weak scattering power of oxygen in the 100 GPa range. Characterisation of the structural changes at the onset of metallisation is essential to understand the mechanism of the insulator-to-metal transition. Advanced single crystal growth techniques at high pressure and temperature and use of optimised conditions for performing microdiffraction are key to unravelling the elusive nature of metallic oxygen.
 |
|
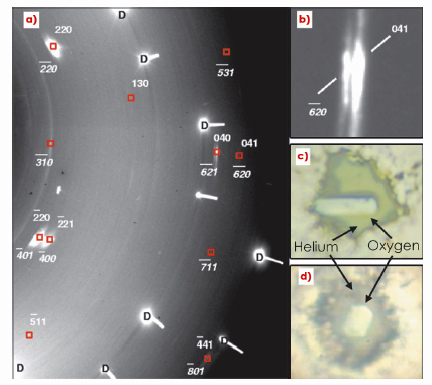
Fig. 11: a) Details of a diffraction image of solid oxygen at 133 GPa, overlaid with the indexing of reflections arising from identified twins, indicated by normal and italic indexing, respectively. b) A magnification of (a), illustrating two different reflections belonging to twin crystals. c) and d) Photomicrographs of two of the oxygen single crystals, oriented differently in surrounding He at 125 GPa in a diamond anvil cell. The field of view is approximately 75 µm. |
To address this problem, we carried out angle-dispersive X-ray diffraction experiments at beamline ID27 on single crystals of solid oxygen embedded in helium, the best hydrostatic pressure transmitting medium. Crystals were grown with different orientations as assessed by the anisotropic optical properties of the ![]() phase (see Figure 11). X-ray diffraction images were collected over the full high pressure cell aperture (2
phase (see Figure 11). X-ray diffraction images were collected over the full high pressure cell aperture (2![]() +/-35°). Below 96 GPa, all observed reflections were well indexed to the known monoclinic C2/m unit cell of the
+/-35°). Below 96 GPa, all observed reflections were well indexed to the known monoclinic C2/m unit cell of the ![]() phase. Upon pressure increase, twinning was reproducibly observed in all crystals. Nevertheless, the crystal mosaicity remained very small up to 96 GPa owing to the good hydrostatic conditions. Upon transition to the metallic
phase. Upon pressure increase, twinning was reproducibly observed in all crystals. Nevertheless, the crystal mosaicity remained very small up to 96 GPa owing to the good hydrostatic conditions. Upon transition to the metallic ![]() phase, diffraction images (Figure 11) showed significant changes characterised by broadening and sliding of the Bragg diffraction spots, the latter being indicative of the displacive nature of the structural transition. The crystalline structure of the metallic
phase, diffraction images (Figure 11) showed significant changes characterised by broadening and sliding of the Bragg diffraction spots, the latter being indicative of the displacive nature of the structural transition. The crystalline structure of the metallic ![]() phase was shown to present the same symmetry and space group (C2/m) as
phase was shown to present the same symmetry and space group (C2/m) as ![]() oxygen, in good agreement with ab initio calculations [3]. This result was corroborated by Raman spectroscopy carried out on single crystals in the
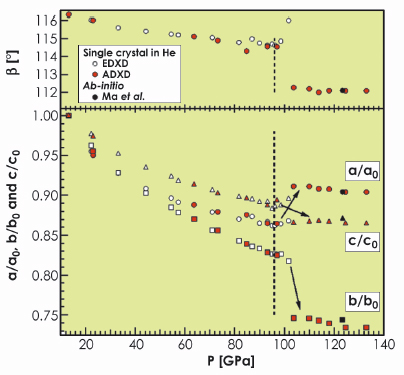
oxygen, in good agreement with ab initio calculations [3]. This result was corroborated by Raman spectroscopy carried out on single crystals in the ![]() phase. The sharp change measured for the monoclinic lattice parameters above 96 GPa, illustrated in Figure 12, indicated a first-order transition taking place essentially in the ab plane. The structural change is characterised by the connection of the O8 units along the b-direction as indicated by the strong contraction of the b-axis between 96 and 106 GPa.
phase. The sharp change measured for the monoclinic lattice parameters above 96 GPa, illustrated in Figure 12, indicated a first-order transition taking place essentially in the ab plane. The structural change is characterised by the connection of the O8 units along the b-direction as indicated by the strong contraction of the b-axis between 96 and 106 GPa.
 |
|
Fig. 12: Pressure dependence of lattice constants a, b, c and angle ß of the C2/m monoclinic unit cell compared with values from a previous energy dispersive X-ray diffraction experiment [4] and ab initio calculations. |
In brief, the present X-ray diffraction and spectroscopic results in combination with the ab initio calculations indicate that the metallisation of solid oxygen takes place by a band gap closure in the ![]() phase and is followed by the destabilisation of the molecular lattice towards the denser structure of the
phase and is followed by the destabilisation of the molecular lattice towards the denser structure of the ![]() phase, in which dissociation of the O8 units occurs.
phase, in which dissociation of the O8 units occurs.
References
[1] L.F. Lundegaard et al., Nature, 443, 201 (2006).
[2] H. Fujihisa et al., Phys. Rev. Lett., 97, 085503 (2006).
[3] Y. Ma, A.R. Oganov and C.W. Glass, Phys. Rev. B, 76, 064101 (2007).
[4] G. Weck et al., Phys. Rev. Lett. 88, 035504 (2002).
Principal publication and authors
G. Weck (a), S. Desgreniers (b), P. Loubeyre (a), and M. Mezouar (c), Phys. Rev. Lett. 102, 255503 (2009).
(a) CEA, Bruyères le Châtel (France)
(b) Laboratoire de physique des solides denses, Université d’Ottawa, Ottawa (Canada)
(c) ESRF



