- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- X-ray Absorption and Magnetic Scattering
- On the occurrence of polymorphism in liquid Sn at high-pressure
On the occurrence of polymorphism in liquid Sn at high-pressure
Solids are known to transform between polymorphs having different structure and physical properties in response to pressure and temperature. The occurrence of analogous density driven phase transitions between two liquids, characterised by different local structures and thermodynamic properties has been the subject of intense interest since a liquid-liquid transition has been discovered in phosphorus (see [1] for a review).
Theoretical models and simulations have shown that liquid-liquid (L-L) phase transitions are possible and most likely to occur as metastable transitions in the undercooled liquid regime. From the experimental side, however, support for the existence of these transformations is still rare, mainly as a consequence of the technical difficulties in probing liquids under metastable and extreme conditions.
In various pure metals, the existence of liquid polyamorphism was inferred by rather sharp changes in the electrical conductivity, specific heat and sample volume. Theoretical studies indicate liquids described by soft-core interatomic potentials (like Ge, Sn, Ga, or Bi), among the possible candidates for exhibiting structural changes in their liquid stable or metastable state.
We further studied the interesting case of liquid Sn, with the aim of clarifying whether the reported anomalies are associated with changes of the local structure and/or nucleation properties.
Using X-ray absorption spectroscopy in combination with complementary techniques, like fixed energy X-ray absorption temperature scans and X-ray diffraction also available at beamline BM29, we have investigated solid and liquid Sn in the 0-4 GPa and 300-850 K pressure and temperature range using a large-volume Paris-Edinburgh apparatus.
From the X-ray absorption temperature scans recorded at different pressures (Figure 103, upper panel) we discovered that above a critical pressure (~ 2 GPa), the undercooling of liquid Sn is drastically reduced by preferential nucleation of the liquid into a solid phase (Sn-III) expected to be stable at higher pressure (~ 3 GPa). This can be observed in Figure 103, where the transition to the Sn-III phase is associated with a sudden increase of the absorption level and the occurrence of diffraction peaks identified as reflections of the body centred tetragonal Sn-III phase.
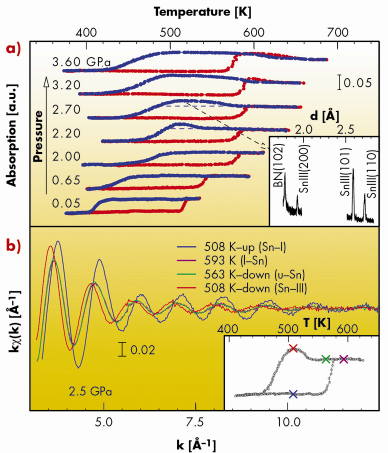 |
|
Fig. 103: a) Series of fixed-energy X-ray absorption temperature scans at different pressures. The drastic changes of the absorption during heating (red) and cooling (blue) ramps correspond to phase transitions. Above 2 GPa, undercooled Sn nucleates in a metastable phase, identified as solid Sn-III by X-ray diffraction (inset). b) XAS k |
In the lower panel of Figure 103, the XAS signals collected at 2.5 GPa, at four different temperatures (marked in the X-ray absorption temperature scan, lower inset) show the close similarity between the local structure of liquid Sn and solid Sn-III.
In order to maximise the structural information that could be extracted from the experimental data, the XAS signals were analysed by Reverse Monte Carlo (RMC) modelling [2]. This approach provides three dimensional models of a disordered system compatible with XAS and diffraction data. These models can be analysed in terms of pair distribution functions, coordination numbers and bond angle distributions, to obtain a complete insight into the atomic correlations on the microscopic level.
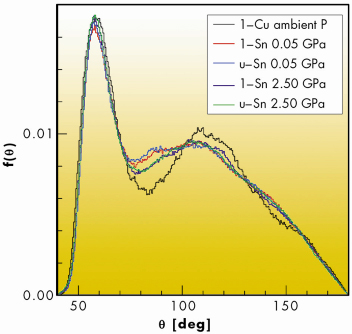
From such analysis, the local structure of liquid Sn is clearly different from that of simple close-packed liquids, such as Cu. In molten Sn, where the bond angle distributions, f(![]() ) in Figure 104, are characterised by a broad hump in the region between 90° and 110°, which includes the tetrahedral angle. A more detailed analysis in terms of local coordination numbers also demonstrates that the total f(
) in Figure 104, are characterised by a broad hump in the region between 90° and 110°, which includes the tetrahedral angle. A more detailed analysis in terms of local coordination numbers also demonstrates that the total f(![]() ) includes contributions from low-coordinated structural units having some degree of tetrahedral ordering, plus close-packed configurations of higher coordination number. Pressure induces a gradual modification of the local structure: tetrahedral-like structural units are progressively reduced upon compression.
) includes contributions from low-coordinated structural units having some degree of tetrahedral ordering, plus close-packed configurations of higher coordination number. Pressure induces a gradual modification of the local structure: tetrahedral-like structural units are progressively reduced upon compression.
 |
|
Fig. 104: Angular distributions of liquid and undercooled Sn at 0.05 and 2.5 GPa, compared with liquid copper at ambient pressure. |
In conclusion, we observed the occurrence of an anomalous phase selection above 2 GPa in liquid Sn. This was found to be accompanied by tiny and gradual changes of the local ordering: upon pressurisation, the local structure evolves toward an arrangement having a closer affinity with a close-packed liquid, but without any sharp modification corresponding to a liquid-liquid phase transition.
References
[1] P.F. McMillan, J. Mater. Chem. 14, 1506 (2004).
[2] A. Di Cicco and A. Trapananti, J. Phys. Cond. Matter 17, S135 (2005).
Principal publication and authors
A. Di Cicco (a), A. Trapananti (b), E. Principi (a), S. De Panfilis (b) and A. Filipponi (c), Appl. Phys. Letters 89, 221912 (2006).
(a) Università Di Camerino (Italy)
(b) ESRF
(b) Università dell’Aquila (Italy)



