- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- Materials Science
- Molecular springs at the onset of life
Molecular springs at the onset of life
How life emerged on Earth remains a mystery to us. Molecules with backbones forming stable double helices held together by Watson-Crick base pairings have an exceptional importance in this saga, in particular because they store genetic information. Many scenarios involve extreme conditions, thus we have undertaken a programme on the behaviour of this particular architecture under high pressure. Our first study was performed on crystals of the octanucleotide d(GGTATACC), where molecules are in the A-DNA form and pack in infinite super-helices of duplexes down the six-fold axis of the P61 space group [1]. In addition to Bragg reflections of the A-form crystal, X-ray diffraction images exhibit a diffuse scattering pattern produced by molecules of the B-DNA forms trapped in the central channel of the super-helix [2]. Accordingly, these crystals gave us the opportunity to monitor the behaviour of both A- and B-forms up to 2.0 GPa (about 20000 atmosphere).
High-pressure macromolecular crystallography was pioneered two decades ago [3]. It has now become a fully-fledged method [4] with a broad range of applications. Crystals are hydrostatically compressed at room temperature in a cavity drilled in a metal gasket that is squeezed between the culets of two diamonds. Pressure is monitored using the wavelength shift of the fluorescence emitted by a ruby sphere deposited in the cavity. Diffraction data are collected using a parallel beam of high energy X-rays (33 keV) and a CCD detector. Special diamond-anvil cells were designed, providing a large useful aperture (82°) as well as a broad pressure range, up to about 2.5 GPa. High completeness diffraction data can be generally collected using few samples, even in cases of crystals with anisotropic habit (e.g. plate) and/or a low symmetry space group [4].
3D-structures of the A-form crystal at ambient pressure, 0.55, 1.09 and 1.39 GPa were refined at 1.6 Å resolution to R (Rfree)-factors of 15.2 (19.1), 16.9 (20.1), 19.3 (22.7) and 18.8 (22.6) %, respectively.
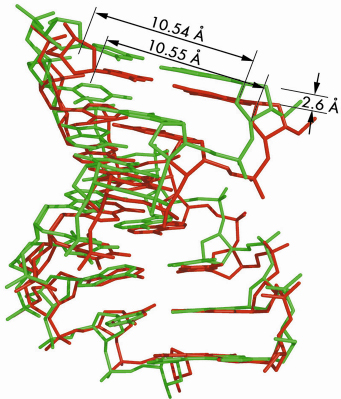
Figure 37 shows the large axial compression (11% at 1.39 GPa) of the molecule. The average base-pair step varies from 2.92 Å down to 2.73 Å. This spectacular plasticity associates with small changes only in phosphodiester backbone angles to accommodate the denser base stacking. The lengths of vectors C1’-C1’, which quantify the DNA cylinder transversal squeeze, are all identical within their standard deviations, whatever the applied pressure. The geometry of Watson-Crick base pairings remains essentially invariant in the pressure domain up to 1.39 GPa.
 |
|
Fig. 37: Superimposition of the A-DNA duplex at ambient pressure (green) and 1.39 GPa (red). The full-duplex length reduces from 23.5 Å at ambient pressure to 20.9 Å at 1.39 GPa. |
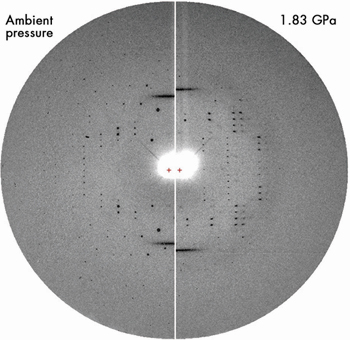
The broad meridional streaks produced by B-DNA octamers occluded within the channels of the crystalline A-form matrix were observed up to at least 2.0 GPa, as shown in Figure 38. The period of B-DNA stacking decreases from 3.40 to 3.10 Å in the range from ambient pressure to 2.0 GPa.
 |
|
Fig. 38: Fibre diagram of B-DNA superimposed on the diffraction pattern of A-DNA crystal at ambient pressure (left) and 1.83 GPa (right). At 1.83 GPa, the two B-DNA meridian reflections still persist over the degrading A-DNA diffraction pattern. The fibre pattern is still observed at 2 GPa (Reproduced with permission from Nucl. Acids Res., 35(14), 4800-4808 (2007)). |
The remarkable adaptation of d(GGTATACC) to high pressure is clearly associated with the base-paired double-helix topology of the molecule, by which it behaves as a molecular spring. These properties are probably shared by molecules featuring a similar topology, with sugar-phosphate or polypeptide backbones. Some of these molecules have catalytic properties and can store genetic information. Such architectures could withstand not only pressure in the deepest sea trenches but also much higher pressures found in Earth’s interior or in the context of rare events such as the impact of a meteorite. We suggest that this remarkable adaptation to harsh conditions may have played a significant role in the selection process during the first steps of the emergence of life on Earth.
References
[1] O. Kennard, W.B.T. Cruse, J. Nachman, T. Prangé, Z. Shakked, D. Rabinovitch, J. Biol. Molec. Struct. and Dynamics, 3, 623-647 (1986).
[2] J. Doucet, J.P. Benoît, W.B.T. Cruse, T. Prangé, O. Kennard, Nature, 337, 190-193 (1989).
[3] C.E. Kundrot, F.M. Richards, J. Mol. Biol., 193, 157-170 (1987).
[4] E. Girard, A.-C.Dhaussy, B. Couzinet, J.-C., Chervin, M. Mezouar, R. Kahn, I. Ascone, R. Fourme, J. Appl. Cryst., 40, 912-918 (2007).
Principal publication and authors
E. Girard (a,g), T. Prangé (b), A.-C. Dhaussy (c), E. Migianu-Griffoni (d), M. Lecouvey (d), J.-C. Chervin (e), M. Mezouar (f), R. Kahn (g), R. Fourme (a), Nucl. Acids Res., 35(14), 4800-4808 (2007).
(a) Synchrotron-SOLEIL, Saint-Aubin (France)
(b) UMR 8015 CNRS, Paris (France)
(c) CRISMAT, ENSICAEN, Caen (France)
(d) UMR CNRS 7033, Bobigny (France)
(e) IMPMC, Paris (France)
(f) ESRF
(g) IBS, UMR 5075 CEA-CNRS-UJF-PSB, Grenoble (France)



