- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2002
- X-ray Absorption and Magnetic Scattering
- Chemistry of the Oxychlorination Catalyst: An In situ, Time-resolved, Dispersive XANES Study
Chemistry of the Oxychlorination Catalyst: An In situ, Time-resolved, Dispersive XANES Study
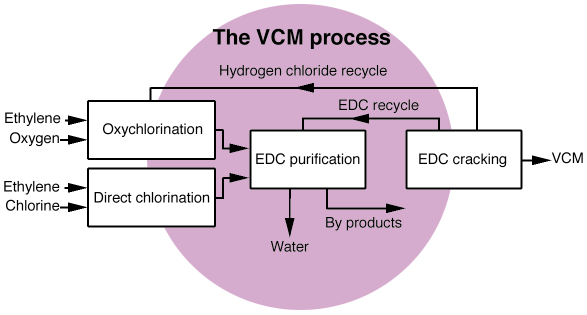
Nowadays, almost all the world's production of PVC (poly vinyl chloride) is obtained by the polymerisation of vinyl chloride (VCM), which is produced using C2H4, Cl2 and O2 as reagents, following the scheme represented in Figure 43. Production of VCM is based on the cracking of C2H4Cl2, which in its turn is produced by two parallel processes, direct chlorination:
C2H4 + Cl2 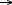 C2H4Cl2 (1)
C2H4Cl2 (1)
and oxychlorination:
C2H4 + 2HCl + 1/2O2 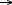 C2H4Cl2 + H2O (2)
C2H4Cl2 + H2O (2)
The oxychlorination reaction (2) allows the HCl produced by the cracking of C2H4Cl2 (EDC) to be recycled, representing a large scale reaction specifically developed to reduce the raw material consumption (Cl2) and to avoid the removal from the cycle of unused reagents (HCl), in agreement with the modern requests of chemical industry. Commercial catalysts are produced by impregnation of  -alumina with CuCl2 (4-8 wt% Cu) following the incipient wetness method [1].
-alumina with CuCl2 (4-8 wt% Cu) following the incipient wetness method [1].
 |
|
Fig. 43: Schematic representation of the industrial production of vinyl chloride (VCM). |
Despite abundant literature on the subject, a significant improvement in the knowledge of the system has been gained only recently [1-3]. By separately feeding in the three reagents, it was shown that the overall ethylene oxychlorination reaction (2) is catalysed by a highly-dispersed CuCl2 phase following a three steps redox mechanism: (i) chlorination of ethylene by reduction of CuCl2 to CuCl, (ii) oxidation of CuCl to an oxychloride and (iii) re-chlorination with HCl (closure of the catalytic cycle) [2,3]:
2CuCl2 + C2H4 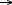 C2H4Cl2 + 2CuCl (3)
C2H4Cl2 + 2CuCl (3)
2CuCl + 1/2O2 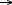 Cu2OCl2 (4)
Cu2OCl2 (4)
Cu2OCl2 + 2HCl 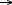 2CuCl2 + H2O (5)
2CuCl2 + H2O (5)
An in situ, time-resolved, XANES study, performed on ID24 has allowed us to determine the Cu2+  Cu+ transformation occurring on the CuCl2/
Cu+ transformation occurring on the CuCl2/ -Al2O3 catalyst in the ethylene oxychlorination reaction atmosphere within the 373-623 K range. This is shown by the derivative spectra reported in Figure 44a and quantified in the red line of Figure 44b). The simultaneous determination of the catalysts activity (blue stars in Figure 44b), has demonstrated that the catalyst works in the reduced state, and that the rate determining step of the reaction (2) is the oxidation of CuCl according to Equation (4).
-Al2O3 catalyst in the ethylene oxychlorination reaction atmosphere within the 373-623 K range. This is shown by the derivative spectra reported in Figure 44a and quantified in the red line of Figure 44b). The simultaneous determination of the catalysts activity (blue stars in Figure 44b), has demonstrated that the catalyst works in the reduced state, and that the rate determining step of the reaction (2) is the oxidation of CuCl according to Equation (4).
 |
|
|
This study has also determined the key role of the potassium chloride dopant, added to the industrial catalysts for fixed-bed reactors, by proving that KCl decreases the rate of the reduction step (3), which becomes the rate-determining step for the KCl/CuClC2/ -Al2O3 catalyst. This dispersive XANES study, supported by parallel IR investigation supports the thesis that the active phase of the industrial catalyst is a non-stoichiometric mixed chloride KXCuCl2+X.
-Al2O3 catalyst. This dispersive XANES study, supported by parallel IR investigation supports the thesis that the active phase of the industrial catalyst is a non-stoichiometric mixed chloride KXCuCl2+X.
References
[1] G. Leofanti, M. Padovan, M. Garilli, D. Carmello, A. Zecchina, G. Spoto, S. Bordiga, G. Turnes Palomino and C. Lamberti, J. Catal. 189, 91-104 (2000).
[2] G. Leofanti, A. Marsella, B. Cremaschi, M. Garilli, A. Zecchina, G. Spoto, S. Bordiga, P. Fisicaro, G. Berlier, C. Prestipino, G. Casali, and C. Lamberti, J. Catal. 202, 279-295 (2001).
[3] G. Leofanti, A. Marsella, B. Cremaschi, M. Garilli, A. Zecchina, G. Spoto, S. Bordiga, P. Fisicaro, C. Prestipino, F. Villain and C. Lamberti, J. Catal. 205, 275-381 (2002).
Principal publication and Authors
C. Lamberti (a,b), C. Prestipino (a,c), F. Bonino (a), L. Capello (a,b), S. Bordiga (a,b), G. Spoto (a), A. Zecchina (b), S. Diaz Moreno (c), B. Cremaschi (d), M. Grilli (d), A. Marsella (d), D. Carmello (d), S. Vidotto (d) and G. Leofanti (d,e), Angew. Chem. Int. Ed. 41, 2341-2344 (2002).
(a) University of Turin (Italy)
(b) INFM UdR of Turin University (Italy)
(c) ESRF
(d) EVC Technological Centre, Porto Marghera, Venezia (Italy)



