- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Electronic structure and magnetism
- A novel solution for the determination of NO oxidation state in Fe-NO complexes
A novel solution for the determination of NO oxidation state in Fe-NO complexes
To understand how the metal iron utilises its intrinsic reactivity and redox propensity for the stabilisation of nitric oxide in the form of Fe-NO complexes, an extensive study of the electronic structure of Fe-NO complexes was carried out using a variety of spectroscopic methods [1-4]. The comparable energy levels of NO π* orbitals and Fe 3d orbitals, however, complicate the Fe-NO bonding interaction and puzzle the quantitative assignment of the oxidation state of Fe-bound nitric oxide as NO+, NO radical, or NO-. With regard to the utilisation of valence-to-core X-ray emission spectroscopy (V2C XES) for quantifying small-molecule activation such as N2 in iron-dinitrogen complexes, we developed V2C XES as a spectroscopic ruler for the quantitative determination of NO oxidation state in Fe-NO complexes, which was circumvented by invention of Enemark-Feltham notation [5].
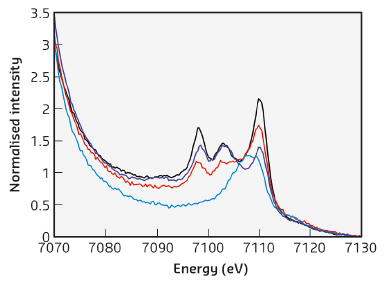
XES displays the dipole-allowed transitions from metal p orbitals to metal 1s orbital after ionisation of a metal 1s electron. The XES valence-to-core region, in particular, reveals the ligand np/ns → metal 1s transitions, which are enhanced by ~5% contribution of Fe 4p orbitals into the valence orbitals. XES spectra of complexes [FeII(SPh)4]2-, [(NO)Fe(SPh)3]-, [(NO)2Fe(SPh)2]-, and [(NO)2Fe(OPh)2]- were collected at beamline ID26. As shown in Figure 19, a broad transition peak at 7107.5 eV is observed in the normalised Fe K-valence spectrum of complex [FeII(SPh)4]2-, as opposed to three distinctive V2C transition peaks at ~7098 eV, ~7103 eV, and ~7110 eV exhibited by nitrosyl iron complexes [(NO)Fe(SPh)3]-, [(NO)2Fe(SPh)2]-, and [(NO)2Fe(OPh)2]-. Presumably, the two additional V2C transition peaks in the lower energy region result from the incorporation of nitric oxide.
 |
|
Fig. 19: Comparison of the normalised Fe K-valence XES spectra of complexes [FeII(SPh)4]2- (blue), [(NO)Fe(SPh)3]- (red), [(NO)2Fe(SPh)2]- (black), and [(NO)2Fe(OPh)2]- (purple). |
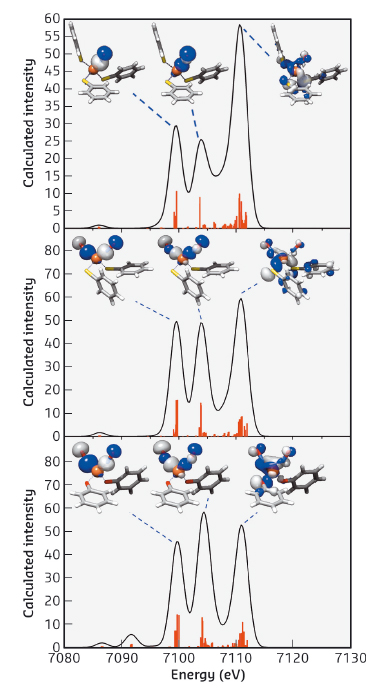
Theoretical calculation was further pursued to verify the nature of these spectral transitions. DFT calculated V2C XES spectra of all Fe-NO complexes resemble the experimental V2C features. DFT calculation provides a computational validation for the dominant contribution from NO σ2s* and σ2p orbitals, respectively, to these XES V2C features (Figure 20). That is, V2C XES probes the energy difference between the σ2s* and σ2p orbitals of Fe-bound nitric oxide. To test the application of this energy difference, ΔEσ2s*–σ2p of NO, to identify the oxidation state of nitric oxide, DFT calculation of ΔEσ2s*–σ2p of NO+, NO radical and NO- was carried out to establish the linear relationship between ΔEσ2s*–σ2p of NO and its oxidation state. The correlation between ΔEσ2s*–σ2p of NO and its oxidation state follows the equation: ΔEσ2s*–σ2p = -0.550 × (oxidation state of NO) + 5.079. Negative correlation between ΔEσ2s*–σ2p of NO and its oxidation state suggests that the addition of electrons into NO π* orbitals weakens N-O bonding interaction, raises the energy level of the σ2p orbital, while reducing that of the σ2s* orbital, therefore resulting in an increase in the energy separation between these two orbitals.
 |
|
Fig. 20: DFT calculated valence-to-core XES spectra of complexes [(NO)Fe(SPh)3]– (top), [(NO)2Fe(SPh)2]– (middle), and [(NO)2Fe(OPh)2]– (bottom) and the corresponding molecular orbitals for each of the transitions. Calculated peaks are displayed in the vertical lines. |
Based on the ΔEσ2s*–σ2p exhibited by Fe-NO complexes [(NO)Fe(SPh)3]–, [(NO)2Fe(SPh)2]–, and [(NO)2Fe(OPh)2]–, the oxidation state of these nitrosyl iron complexes are –0.58±0.18, –0.77±0.18, and –0.95±0.18, respectively. This finding suggests that binding of nitric oxide toward non-heme iron, for the sake of the storage and transport of nitric oxide, affords Fe-NO complexes and stabilises NO via partial reduction. We aim to expand this spectroscopic method to quantify NO oxidation state in a variety of Fe-NO complexes and to bring insights into Fe-NO bonding interaction modulated by its geometric structure and supporting ligands in the near future.
Principal publication and authors
T.-T. Lu (a), T.-C. Weng (b) and W.-F. Liaw (c), Angew. Chem. 53, 11562 (2014).
(a) Department of Chemistry, Chung Yuan Christian University (Taiwan)
(b) SLAC National Accelerator Laboratory (USA)
(c) Department of Chemistry, National Tsing Hua University (Taiwan)
References
[1] T.-T. Lu et al., Inorg. Chem. 50, 5396 (2011).
[2] J.L. Hess, C.H. Hsieh, S.M. Brothers, M.B. Hall and M.Y. Darensbourg, J. Am. Chem. Soc. 133, 20426 (2011).
[3] S. Ye, F. Neese, J. Am. Chem. Soc. 132, 3646 (2010).
[4] J.E.M.N. Klein et al., Angew. Chem. 53, 1790 (2014).
[5] J.H. Enemark and R.D. Feltham, Coord. Chem. Rev. 13, 339 (1974).



