- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structure of materials
- Hydration of a Nafion membrane in a working fuel cell
Hydration of a Nafion membrane in a working fuel cell
Proton exchange membrane fuel cells convert chemical into electrical energy. They are promising candidates for the development of an environmentally-friendly hydrogen-based technology. The fuel cell’s performance strongly depends on the degree of hydration of the proton exchange membrane, since proton transfer in such polymeric materials is known to be assisted by water.
Here, a time-resolved vertical stratigraphy of the proton exchange membrane (PEM) was performed, slicing the membrane into a stack of virtual layers from one electrode to the other. The time dependence of the hydration degree in each layer was determined with the highest accuracy ever achieved for the characterisation of such a fuel cell under typical working conditions (polarisation curve) [1]. The same technique could easily be applied to cells operating under different conditions, thus providing a powerful tool to investigate the fundamental relationships between hydration degree and electrochemical performance.
The penetrating power of the high energy radiation at beamline ID15 allowed in situ XRD measurements on a test fuel cell with a design equivalent to that of a standard cell, avoiding artefacts due to the modifications usually required to measure working devices.
A membrane electrode assembly with Pt nanoparticles (20% Pt/Vulcan XC-72 powder from E-TEK, Pt loading about 1 mg/cm2) as anode and cathode catalysts, and Nafion® as proton conducting membrane, was used as the active element.
A fuel cell is far from an ideal system on which to perform diffraction measurements since both the membrane’s amorphous polymers and the water contained within it provide rather weak scattering signals. However, the use of high energy radiation coupled with the adoption of an image plate detector resulted in a fine time sampling of the water dynamics in each layer of the PEM.
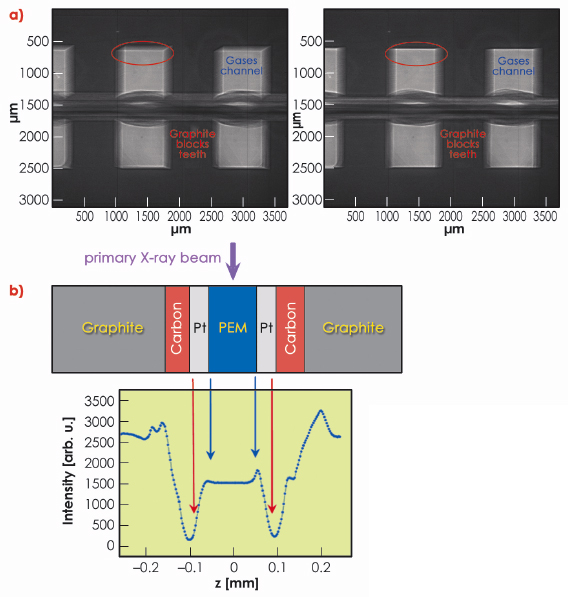
Positioning and alignment of the cell was done by taking a sequence of radiographs during a rocking-angle scan (Figure 42a). This also permitted identification of the cell components. Once the cell had been aligned, a subsequent transmission measurement with a narrow X-ray beam through the proton exchange membrane and its adjacent cell components allowed details of the membrane electrode assembly to be finely resolved. (Figure 42b).
 |
|
Fig. 42: a) X-ray radiography of the fuel cell. Left: after preliminary row positioning, right: after alignment to the primary X-ray beam. b) X-ray transmission measurements through the membrane electrode assembly. |
Time/space-resolved sequences of diffraction patterns were collected during a polarisation curve (40 mA, steps of 30 minutes duration) from 0 to 1 A (insert of Figure 43). The primary
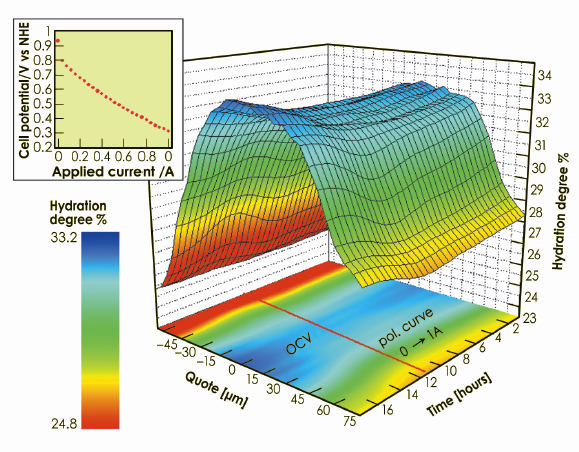
X-ray beam cross section was 100 x 5 µm2 (horizontal x vertical), the latter corresponding to the spatial resolution which allowed the hydration degree of 31 slices of the membrane to be investigated independently. The information on the quantity of water contained in a portion of membrane was obtained by applying the spectral decomposition method [2]. Several scans were also executed under open circuit conditions after the end of the polarisation, to follow the hydration behaviour during cell relaxation. The rate of change of the cell current was slow enough to consider the membrane to be in quasi-equilibrium conditions during the whole experiment (quasi static process). The result of the time/space-resolved investigation is reported in the 3D plot in Figure 43, which represent a compact summary of the entire information obtainable on the hydration dynamics in the PEM.
 |
|
Fig. 43: Space/time-resolved study of the water distribution in the proton exchange membrane. 3D plot of the time-dependent water content in each “slice” of the membrane, carried out under real working conditions (polarisation curve in the inset, followed by an open circuit period). The iso-level projection of the surface on the basal plane is also reported. |
The present study paves the way to systematic observations of the water management in any kind of polymeric material (both pure and nanoparticle filled), used as a solid electrolyte in proton exchange membrane fuel cells. The method used has both sufficient spatial and temporal resolution to follow the water distribution within a working fuel cell membrane.
Acknowledgements
This research was partially supported by the NUME project (Italian Ministry of University and Research, FISR 2003).
References
[1] J. St-Pierre, J. Electrochem. Soc 157, 7 B724 (2007).
[2] V. Rossi Albertini, B. Paci, A. Generosi, S. Panero, M. A. Navarra and M. Di Michiel, Electrochem. Solid-State Lett 7, A519J (2004).
Principal publication and authors
V. Rossi Albertini (a), B. Paci (a), F. Nobili (b), R. Marassi (b) and M. Di Michiel (c), Advanced Materials 21, 578 (2009).
(a) Istituto di Struttura della Materia, C.N.R, Roma (Italy)
(b) Dipartimento di Scienze Chimiche, Università di Camerino, Camerino (MC) (Italy)
(c) ESRF



