- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- High resolution and resonance scattering
- X-ray absorption of ordered and disordered ice
X-ray absorption of ordered and disordered ice
Despite extensive experimental and theoretical investigations over many decades, an unambiguous understanding of the structure and nature of the hydrogen bonding of water in disordered condensed phases remains an elusive goal [1]. The absorption spectrum of the core 1s oxygen (oxygen K-edge X-ray absorption XAS) is a sensitive atom-specific probe for the H-bond local environment around the absorbing atom. From the comparison of oxygen 1s electronic excitations near the absorption threshold (pre-edge) with theoretical calculated spectrum of liquid water, it was postulated that there exists a large fraction of water molecules with broken hydrogen bonds [2]. This hypothesis challenges the conventional model of liquid water where each water molecule is approximately tetrahedrally coordinated to four neighbouring water molecules through hydrogen bonds (H-bond). The basic premise for this suggestion is the observation of a strong pre-edge absorption feature analogous to water adsorbed on surfaces with a broken H-bonding network.
To characterise the nature of the distinct pre-edge absorption, oxygen K-edge XAS of several ice polymorphs were studied using the method of X-ray Raman spectroscopy (XRS). Using X-ray radiation with energy much higher than the core ionisation, XRS measures energy losses due to electronic excitations in a method that is analogous to XAS. Thanks to the higher penetration depth of the X-ray, it is essentially a bulk technique and not affected by surface and saturation effects usually encountered in soft X-ray absorption. To ensure direct and unbiased comparison of different spectra, XRS measurements were made on several ice polymorphs under almost identical instrument resolution, energy calibration and sample environment. This was achieved by taking advantage of the well-characterised thermal induced in situ successive structural phase transformation of a high-density amorphous ice (HDA) to low density amorphous (LDA), to crystalline ice Ic and ice Ih and eventually to liquid water [3]. Oxygen K-edge XRS spectra were recorded at beamline ID16. It was imperative that the sample was at a well-defined temperature; therefore, experiments were carried out at 40 K with the sample bathed in cold flowing He gas. Radiation damage to the sample was minimised by shortening the collection time (less than 1 minute) and accumulating the spectra at multiple sites on the sample.
 |
|
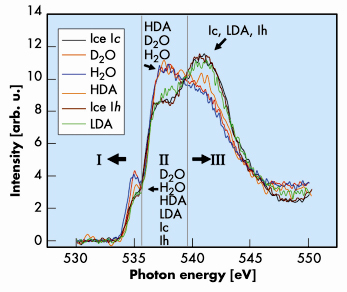
Fig. 8: X-ray Raman spectra for LDA, HDA, ice Ic, ice Ih, H2O, and D2O water. The spectra were normalised to equal area up to 550 eV. The XAS spectra can be divided into three regions: (I) pre-edge, (II) near-edge, and (III) post-edge. The order of the structures starting from the top peak in the pre-edge region is indicated in the lower central part of the figure. The structures that are in the highest peaks in regions II and III are indicated in each of these regions. |
Figure 8 compares the experimental XAS for the various forms of ice and water. In general, the oxygen K-XAS can be divided into three regions. A pre-edge region (I) starts from the absorption threshold at 533 to 536 eV. A near-edge region (II) from 537 to 539 eV, and a post-edge region (III) from 539 eV and beyond. All the ice samples show a pre-edge absorption at 535 eV. Moreover, the intensity of this peak decreases in the order from liquid water to HDA, and is almost the same for cubic ice Ic, hexagonal ice Ih, and LDA. In addition, an unusual trend is observed in regions II and III where the XAS can be divided into two distinct groups with similar absorption profiles. The first group consists of water and HDA ice (group A) and the second group consists of ice Ic, ice Ih, and LDA (group B). In the near-edge region (II) the intensities of group A are stronger than group B but the reverse is observed in the post-edge region (III). Comparison of the XAS profile for groups A and B suggests that the oscillator strengths of the excitations in the near-edge region for group B have been shifted into the post-edge region.
The experimental results show that core-hole excitations are localised and not strongly affected by the local environment. The observed XAS profiles can be correlated to the degree of the distortion of H-bond network of the ice polymorphs. In highly disordered ices, the intensity of the XAS is stronger near the oxygen-K absorption edge. In contrast, for structurally more ordered ices, the XAS absorption intensity is distributed beyond the absorption edge. The difference in the absorption profile can be interpreted as an enhancement of Wannier over Frenkel excitations in an ordered crystal. The results further suggest important contributions from continuum electronic states which have been neglected in most theoretical calculations. Finally, the existence of the pre-edge feature is not a concise indicator of the magnitude of local disorder within the hydrogen bonded network.
Principal publication and authors
J.S. Tse (a), D.M. Shaw (a), D.D. Klug (b), S. Patchkovskii (b), G. Vankó (c), G. Monaco (c), M. Krisch (c), Phys. Rev. Lett. 100, 095502 (2008).
(a) Physics and Engineering Physics, University of Saskatchewan, Saskatoon (Canada)
(b) Steacie Institute for Molecular Sciences, National Research Council of Canada, Ottawa (Canada)
(c) ESRF
References
[1] Thematic issue on Water, edited by L.R. Pratt, Chem. Rev. 102, 2625 (2002).
[2] Ph. Wernet et al., Science 304, 995 (2004).
[3] C.A. Tulk et al., Science 297, 1320 (2002).



