- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- X-ray Imaging and Optics
- High-resolution mapping of human breast tissue samples and comparison with their histopathology
High-resolution mapping of human breast tissue samples and comparison with their histopathology
Computed tomography (CT) could be extremely important in breast cancer diagnosis, for localising a tumour and revealing its size and inner structure. Conventional whole-body CT scanners do not have sufficient spatial resolution, and the radiation dose is excessive. High-resolution scanners, with an instrumental resolution of about 0.4 mm, are changing this situation, although their accessible domain of spatial resolution and image contrast remains limited by the allowed dose. The attainable resolution is at the limit of revealing essential morphological features of a small-size tumour.
Refraction and scattering contrast are utilised in analyser-based X-ray imaging (ABI), where perfect crystal X-ray optics is used for conversion of small changes in the propagation direction to intensity changes [1]. The contours of the areas where the tissue density changes are emphasised, revealing the morphology of the object. Here we report on the investigation of human breast samples (tumorous and non-tumorous) with the purpose of demonstrating the clinical importance of ABI-CT in mammography.
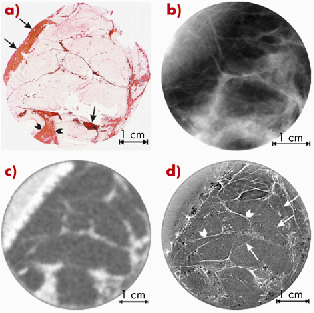
Figure 126 shows a series of images of a surgically-removed breast sample, which was affected by a bifocal ductal carcinoma. In Figure 126a there is a histological section, which was used as a standard for interpretation of findings. A planar screen-film mammogram taken at the hospital is shown in Figure 126b. The CT image acquired with a clinical scanner (Figure 126c) had a spatial resolution of about 0.7 mm. The synchrotron radiation ABI-CT image (TOP) shown in Figure 126d was acquired at ID17 using a taper optics Frelon camera [2] with a spatial resolution of more than one order of magnitude better (0.047 mm) and using the same dose as for the clinical scanner. This image shows a stronger contrast and has a much higher spatial resolution than Figure 126b; the collagen-rich areas are identified by rapid variations of intensity, which correspond to the orientation of the collagen fibres.
 |
(Reprinted from High-resolution CT by diffraction-enhanced X-ray imaging: mapping of breast tissue samples and comparison with their histo-pathology A. Bravin, J. Keyriläinen, M. Fernández, S. Fiedler, C. Nemoz, M-L. Karjalainen-Lindsberg, M. Tenhunen, P. Virkkunen, M. Leidenius, K. von Smitten, P. Sipilä and P. Suortti, Phys. Med. Biol. 52 (8) 2197-2211 (2007), copyright 2007, with permission from IOP Publishing Ltd.) |
|
Fig. 126: (a) Scanner image of the histologic whole-mount slide from the centre plane of 24 mm thick sample with ductal carcinoma. The nuclei are shown in brown or black, young collagen and reticulin in bluish, mature collagen in red, adipocytes in white and both red blood cells and muscle in a yellow colour; (b) clinical screen-film mammogram of the same sample (26 kV, 5.0 mAs); (c) clinical CT image of the same sample (80 kVp, 50 mAs); (d) ABI-CT TOP image of the same sample (33 keV). |
|
The results of this study show that ABI-CT images acquired with a high-resolution detector permit a significant improvement in the visualisation of the morphology and of the overall architecture of the breast tissues. All ABI-CT images show an enhanced contrast [1] and many details become visible that would otherwise be radiotransparent with both planar and CT imaging with conventional sources. The one-to-one correspondence of the ABI-CT image with the corresponding optical image of the stained histology section is evident. The independent observations by the pathologist and the radiologist involved in this study identified the same features in the histological sections and in the ABI-CT images, and they arrive at very similar conclusions.
Earlier studies highlighted that small-angle X-ray scattering (SAXS) from collagen, which is rejected by the analyser at the TOP position, is much stronger than scattering from the adipose tissue [3]. In the areas where cancer has invaded, the molecular structure of collagen differs from the structure of normal ‘healthy’ collagen [3,4]. SAXS from collagen in these areas is stronger than scattering from normal collagen. Further studies are needed to show whether this difference is sufficiently large to be observed in the TOP images.
The next step in ABI-CT is the reconstruction of volume images from the two-dimensional images. The goal is to produce a high-resolution 3D image of the tumour that can be arbitrarily rotated on the computer screen and inspected visually. In principle, the ABI method can be imported to the clinical environment because the refraction and scattering contrast in analyser-based imaging do not require the full coherence of the synchrotron beam. On the other hand, compact SR sources that operate in the hard X-ray regime are being developed, and these will be ideally suited to many medical applications including ABI.
References
[1] A. Bravin, J. Phys. D: Appl. Phys. 36, A-24-29 (2003).
[2] P. Coan, A. Peterzol, S. Fiedler, C. Ponchut, J.C. Labiche and A. Bravin J. Synch. Rad 13, 260-270 (2006).
[3] M. Fernández, J. Keyriläinen, R. Serimaa, M. Torkkeli, M-L. Karjalainen-Lindsberg, M. Tenhunen, W. Thomlinson, V. Urban and P. Suortti Phys. Med. Biol. 47, 577–92 (2002).
[4] M. Fernández, J. Keyriläinen, R. Serimaa, M. Torkkeli, M.-L. Karjalainen-Lindsberg, M. Leidenius, K. von Smitten, M. Tenhunen, S. Fiedler, A. Bravin, T.M. Weiss and P. Suortti Phys. Med. Biol. 50, 2991–3006 (2005).
Principal publication and authors
A. Bravin (a), J. Keyriläinen (a, b), M. Fernández (a, c), S. Fiedler (a, d), C. Nemoz (a), M-L. Karjalainen-Lindsberg (a), M. Tenhunen (b), P. Virkkunen (a), M. Leidenius (a), K. von Smitten (a), P. Sipilä (h) and P. Suortti (a, c) Phys. Med. Biol. 52, 2197–2211 (2007).
(a) ESRF
(b) Helsinki University Central Hospital (Finland)
(c) Department of Physical Sciences, University of Helsinki (Finland)
(d) European Molecular Biology Laboratory, Hamburg (Germany)
(h) Radiation Metrology Laboratory, Radiation and Nuclear Safety Authority (Finland)



