- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- X-ray Imaging and Optics
- Iron storage within dopamine neurovesicles revealed by X-ray fluorescence nano-imaging
Iron storage within dopamine neurovesicles revealed by X-ray fluorescence nano-imaging
Parkinson’s disease results from a shortage of dopamine in the brain induced by the selective death of dopamine producing neurons in the substantia nigra. Dopaminergic neurons die in a slow but progressive manner leading to a depletion of dopamine in the striatum compromising the capacity of the brain to orchestrate voluntary movement. The causes of the selective death of dopaminergic neurons are still largely unknown. Increasing evidence suggests that abnormal iron handling in the brain may be involved in the etiology of Parkinson’s disease [1]. Indeed, iron specific accumulation in the substantia nigra is associated with Parkinson’s disease [2]. It has been suggested that dopamine may exert a protective effect by chelating iron in dopaminergic neurons and that this system might be at fault in Parkinson’s disease. It is therefore theoretically possible that dopamine-iron complexes may exist in dopaminergic neurons but they have not yet been evidenced experimentally.
 |
|
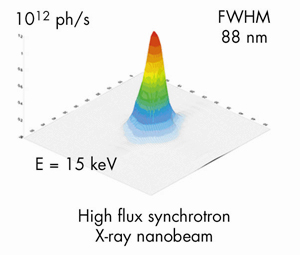
Fig. 121: The intensity distribution in the focal plane of the X-ray fluorescence nanoprobe end-station installed on ID22 beamline. |
Up to now the lack of analytical techniques with sufficient spatial resolution and detection sensitivity prevented the study of iron distribution in neurons at the subcelullar level. An original setup for high spatial resolution chemical imaging has been developed on ID22 beamline with a 88 nm X-ray beam (Figure 121) of very high flux, up to 1012 photons/s. The characteristics of this X-ray nanoprobe fulfil the requirements for mapping biological trace element distributions at a size compatible with the analysis of most cellular compartments such as mitochondria, lysozomes, or neurosecretory vesicles. As exemplified in Figure 122, this newly developed synchrotron X-ray fluorescence nanoprobe is able to detect down to 10–18 g of Fe within a cellular structure as small as 100 nm diameter.
 |
|
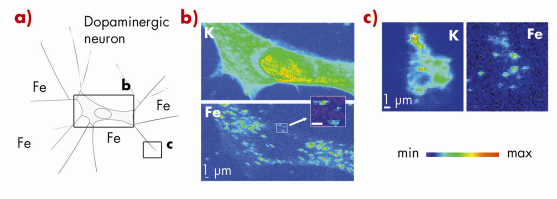
Fig. 122: Synchrotron X-ray fluorescence nano-imaging reveals iron sub-cellular distribution. a) dopamine producing cells were exposed in vitro to iron. Chemical element distributions, here potassium and iron, were recorded on distinct cellular areas such as b) cell bodies, and c) neurite outgrowths, and distal ends. Iron was found in 200 nm structures in the cytosol, neurite outgrowths, and distal ends, but not in the nucleus. Min-max range bar units are arbitrary. Scale bars = 1 µm. |
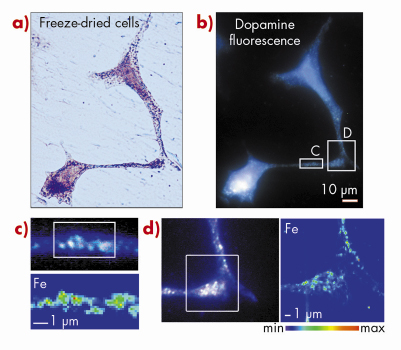
Using this X-ray fluorescence nano-imaging system we investigated the subcellular distribution of iron in dopamine producing neurons. PC12 rat pheocromocytoma cell line was used as an in vitro model of dopamine producing cells. The study shows that iron accumulates into dopamine neurovesicles (Figure 123). In addition, the inhibition of dopamine synthesis results in decreased vesicular storage of iron. These results indicate a new physiological role for dopamine in iron buffering within normal dopamine producing cells. They also suggest that the elevation of iron concentration in the substantia nigra of Parkinson’s disease patients may lead to a lack of iron-dopamine binding capability rendering the dopaminergic neurons more prone to iron toxicity. It has been suggested that mutations in a-synuclein, a protein mutated in some familial forms of Parkinson’s disease, could result in a reduced number of vesicles being available for dopamine storage, leading to an accumulation of dopamine in the cytoplasm and increased levels of oxidative stress [3]. In this context, a mechanism involving iron in Parkinson’s disease progression could result from the redistribution of highly oxidant iron-dopamine compounds in dopaminergic neurons. Using synchrotron X-ray fluorescence nano-imaging it is now possible to investigate the subcellular distribution of metal ions involved either in pathological or physiological functions.
 |
|
Fig. 123: Iron is localised within dopamine neurovesicles. a) Visible light microscopy of freeze-dried cells. b) Epifluorescence microscopy of the same freeze-dried cells enables the identification of dopamine distribution. c, d) Synchrotron X-ray fluorescence nano-imaging reveals the distribution of iron. Dopamine and iron are co-located within 200 nm structures characteristic of dopamine neurovesicles as identified by epifluorescence microscopy. A large number of iron and dopamine neurovesicles are found in c) neurite outgrowths and d) distal ends. Min-max range bar units are arbitrary. Scale bars = 1 µm. |
References
[1] E. Bossy-Wetzel, R. Schwarzenbacher, S.A. Lipton, Nature Med. 10, S2 (2004).
[2] D.T. Dexter, F.R. Wells, F. Agid, Y. Agid, A.J. Lees, et al., Lancet 2, 1219 (1987).
[3] H.A. Lashuel, H. Hirling, ACS Chem. Biol. 1, 420 (2006).
Principal publication and authors
R. Ortega (a), P. Cloetens (b), G. Devès (a), A. Carmona (a,c), S. Bohic (b,d), PLoS ONE 2, e925 (2007).
(a) CNRS-Universités de Bordeaux 1 et 2, Chimie Nucléaire Analytique Bioenvironnementale, Gradignan (France)
(b) ESRF
(c) Universidad de Sevilla, Centro Nacional de Aceleradores, Sevilla (Spain)
(d) INSERM, Institut des Neurosciences, Equipe 6, Grenoble (France)



