- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- X-ray Absorption and Magnetic Scattering
- Newly-developed in situ Time-resolved Spectroscopic Techniques to Reveal Homogeneous Catalytic Reaction Mechanisms
Newly-developed in situ Time-resolved Spectroscopic Techniques to Reveal Homogeneous Catalytic Reaction Mechanisms
The formation of aryl-aryl bonds and aryl-hetero-atom bonds are among the most important reactions in organic synthesis. Caryl-N containing structures are important in biological systems and are common moieties in pharmaceuticals. Although in many cases palladium is a more active and selective catalyst under milder conditions compared to copper, some reactions only proceed with copper. Moreover, because of its lower costs, copper is preferred for industrial processes. The mechanisms proposed for the Cu catalysed reactions are as yet reasonable guesses, because none of the intermediates have been isolated or characterised. Detailed structural information about catalysts in their active phase is in general the missing link in explaining structure-performance relationships in homogeneous catalysis.
X-ray absorption fine structure (XAFS) spectroscopy is a powerful technique for determining the local structure of metal complexes, such as the type and number of neighbouring atoms, distances and electronic properties. XAFS can be applied in situ and, using an energy dispersive data acquisition setup (ED-XAFS), spectra can be obtained in the sub-second range during reaction. With the use of ultraviolet and visible absorption spectroscopy (UV-Vis), the electronic absorption by molecules can be detected in situ and with time-resolution.
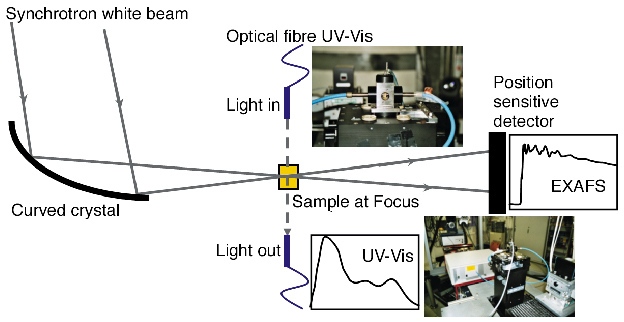
In collaboration with the ESRF staff at ID24, we have developed a novel setup (Figure 102) which enables the simultaneous acquisition of time-resolved in situ XAFS and UV-Vis data on homogeneous catalytic systems. A stopped-flow module is used to perform the reactions. Special cuvettes have been designed, in which the X-rays and UV-Vis light traverse perpendicular to one another. Different path lengths enable the simultaneous ED-XAFS and optical fibre UV-Vis experiments on the reaction mixture.
 |
|
Fig. 102 Schematic representation of the combined ED-XAFS/UV-Vis setup. The insets display the complete setup and the cuvette in the observation head of the stopped flow module. |
The Cu(II)-catalysed arylation reaction of phenylboronic acid and imidazole to N-phenylimidazole was studied at ambient conditions, with a time resolution of 10-200 milliseconds. Stoichiometric reactions were performed to decrease the number of reaction intermediates. The reactions were conducted using 20 mM of the dinuclear tetramethylenediamine bis-µ-hydroxy copper(II) complex [Cu(OH)(TMEDA)]2Cl2 as homogeneous catalyst.
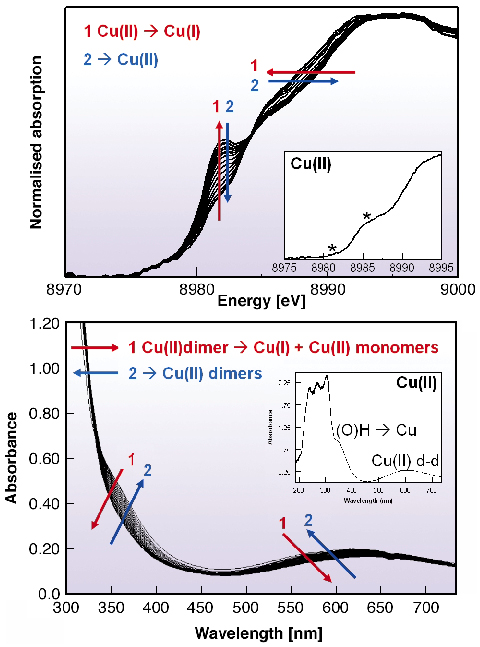
In Figure 103 examples of the simultaneous ED-XAFS and time-resolved UV-Vis spectra of the arylation reaction are shown. The dinuclear Cu(II) structure of the starting Cu catalysts is confirmed. During reaction, the starting Cu(II) peaks disappear and an immediate formation of a pre-edge at ~8982 eV (Figure 103a) indicates the formation of a Cu(I) species. Simultaneously, UV-Vis spectra show the disappearance of the band at ~355nm and the decrease of the d-d transition band at ~620 nm, i.e. the dissociation of copper dimers and the disappearance of Cu(II) species respectively. These spectroscopic results thus suggest the formation of a monomeric Cu(I) species. In time, both ED-XAFS and UV-Vis results display the disappearance of Cu(I) species and the regeneration of Cu(II) species, both in monomeric and dimeric form.
 |
|
Fig. 103: Simultaneous (a) EDXAFS and (b) UV-Vis spectra of the Cu(II) catalysed arylation reaction. The insets show the starting dimeric Cu(II) catalyst in solution. |
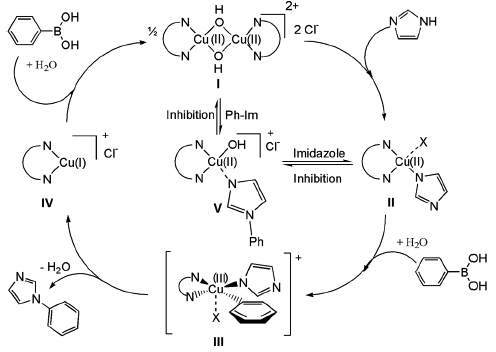
Single step reactions were performed to mimic subsequent individual steps in the catalytic cycle and using these complementary techniques, the different reaction intermediates have been characterised in detail. In combination with catalytic results, a novel and unexpected mechanism for the Cu(II) catalysed arylation reactions has been proposed, Figure 104, which is in accordance with the simultaneously acquired ED-EXAFS results and with results from other characterisation techniques (e.g. EPR, NMR, XRD).
 |
|
Fig. 104: Proposed reaction mechanism for the Cu(II) catalysed arylation of imidazole and phenylboronic acid. |
he first and selectivity determining step in the catalytic cycle is the reaction of the Cu(II) dimer with imidazole, forming a monomeric Cu(II)(imidazole) intermediate. Upon subsequent addition of the phenylboronic acid reactant, a transient Cu(III) intermediate is formed which after reductive elimination forms the phenylimidazole product and a Cu(I) monomeric species. Finally, the Cu species is reoxidised forming again the Cu(II) mononuclear and dinuclear complexes. Inhibition by imidazole and phenylimidazole is observed. The phenylboronic acid is, in combination with H2O, involved in the oxidation and reoxidation steps, whereas O2 is not necessary for the reaction to proceed.
In summary, we have developed a new combined spectroscopic setup, which is a powerful tool for the in situ and time-resolved characterisation of reaction intermediates and therefore for the determination of reaction mechanisms. In principle every metal can be studied, however, the time resolution and quality of the data are dependent on the metal and its concentrations used.
Principal Publication and Authors
M. Tromp (a), G.P.F. van Strijdonck (b), S.S. van Berkel (b), A. van den Hoogenband (c), M.C. Feiters (d), B. de Bruin (d), S.G. Fiddy (e), A.M.J. van der Eerden (a), J.A. van Bokhoven (a), P.W.N.M. van Leeuwen (b), D.C. Koningsberger (a), submitted.
(a) Utrecht University (Netherlands)
(b) University of Amsterdam (Netherlands)
(c) Solvay Pharmaceuticals (Netherlands)
(d) University of Nijmegen (Netherlands)
(e) ESRF



