- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2002
- High Resolution and Resonance Scattering
- Molecular Spin Transitions Studied with X-ray Emission Spectroscopy (XES)
Molecular Spin Transitions Studied with X-ray Emission Spectroscopy (XES)
Making use of isolated molecules in electronic devices is expected to bring major advances to information technology. Photoswitchable molecular magnets, for example, have potential applications in data storage and display devices. The most promising candidates are the so-called spin transition complexes, which are coordinate compounds of transition metal ions with medium ligand-field strength and an electronic configuration between d4 and d7. In these systems, the spin state of the molecules can be switched back and forth between the low spin (LS) and the high spin (HS) states by triggering a redistribution of the 3d electrons on the t2g and eg orbitals with external perturbations such as variation in the temperature or pressure, as well as irradiation with light [1].
Recent research work on these materials is concerned with systems containing two or more different metal sites and their behaviour under extreme conditions like high pressure. With such experiments, new challenges are to be faced due to the possible presence of different metal ions and sample environments with limited access to standard techniques. Therefore, the field would certainly profit from element-sensitive spectroscopic probes of the local (atomic) magnetism and the electronic state, which make use of penetrating radiation. We have studied spin transition complexes of Fe3+, Fe2+ and Co2+ (well below and above their transition temperatures) on ID26 to demonstrate that techniques based on X-rays emitted after 1s core ionisation of the metal ions [2] have these attributes.
The studied compounds, which cover almost the entire d4 to d7 range, have unambiguous spin states and show diverse spin transitions. These thermally-induced transitions were found to affect all features of the X-ray emission spectra; nevertheless, the largest variations were observed on the Kß lineshapes, which arise from the 3p to 1s transition. In Figure 71 we show that the intensity of the satellite peak on the low energy side of the main line follows the variations in the spin state of the molecule. In fact, the satellite intensity is related to the local magnetic moment on the 3d orbitals, through the exchange interaction of the latter and the 3p core hole of the final state. Theoretical calculations were also made, and the calculated lineshapes were in agreement with the experimental data. The Kß spectra reflect the variation of the spin states of different transition metal ions of well-defined 3d configurations with preserved chemical environment and valence state. They can be regarded as benchmark measurements in studying magnetism with XES.
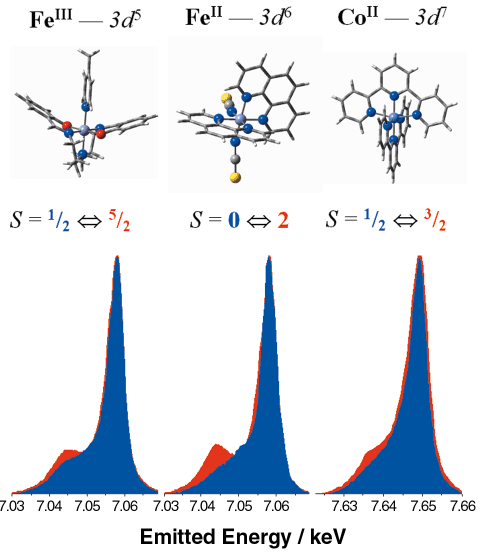 |
|
Fig. 71: Electronic configuration, structure, spin states and Kb spectra of the studied complexes. The intensity of each spectra is normalised to its maximum. The peaks are shifted for better comparision. (Colour code for the spectra: blue - LS, red - HS). |
As the rearrangement of the electrons at the spin transition affects the electronic structure and the molecular geometry, X-ray absorption spectroscopy (XAS) can also be used to follow the transition. However, better separation of XAS spectral features can be achieved by selective detection of emitted X-rays using a high-resolution spectrometer with the analyser energy fixed to a maximum of the emission spectra. We applied this detection technique, called partial fluorescence yield (PFY) with the analyser energy fixed to the Ka1 emission line, to record the X-ray absorption near edge structure (XANES) spectra for the different spin states. In the case of Fe2+ the variations of the molecular geometry are dramatic and completely alter the X-ray absorption spectra (Figure 72a). On the other hand, the Co2+ compound is known for having the smallest changes in the bond length among all spin transition molecules. Accordingly, the PFY-XANES spectra show relatively small changes as displayed in Figure 72b. However, the variation of the pre-edge structure, where contributions from 1s to 3d quadrupolar transitions are expected, is remarkable due to the differences of the electronic distribution on the 3d orbitals.
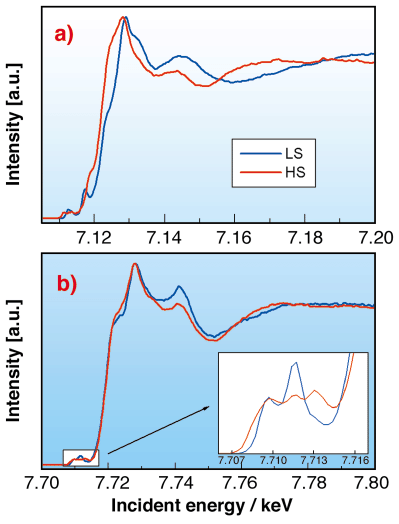 |
|
Fig. 72: Partial fluorescence yield XANES spectra (taken on the K |
Furthermore, in the transition regime both the Kß and the pre-edges of the PFY-XANES spectra were shown to be superpositions of the HS and LS states. This allows convenient determination of the HS fraction and thus the monitoring of the spin transition by either technique. This work should stimulate the use of XES and PFY-XANES in numerous chemical studies, particularly in the field of molecular magnetism.
References
[1] P. Gütlich, A. Hauser, H. Spiering, Angew. Chem. Int. Ed. Engl. 33, 2024-2054 (1994), and references therein.
[2] F.M.F. de Groot, Chem. Rev. 101, 1779-1808 (2001), and references therein.
Authors
G. Vankó (a), T. Neisius (a), F. Renz (b), S. Kárpáti (c), A.Shukla (d), A. Mirone (a), F. de Groot (e).
(a) ESRF
(b) Gutenberg Univ., Mainz (Germany)
(c) Eötvös Loránd Univ. Budapest (Hungary)
(d) LMCP, Univ. Pierre et Marie Curie, Paris (France)
(e) Utrecht University, Utrecht (The Netherlands)
 1 emission line) of a) the [FeII(phen)2(SCN)2] and b) the [Co(terpy)2]2+ complexes. The inset shows the pre-edge region.
1 emission line) of a) the [FeII(phen)2(SCN)2] and b) the [Co(terpy)2]2+ complexes. The inset shows the pre-edge region.


