- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Microfocussing and Imaging
- X-ray Microfluorescence Imaging of Single Cells
X-ray Microfluorescence Imaging of Single Cells
Spatial distribution and concentration of trace elements in tissues are of importance as they are involved in many biological functions of living organisms such as metabolism and nutrition. The importance of the microanalyses of individual cells has been reviewed by Llabador and Moretto [1] who emphasised the future importance of microprobe techniques for certain cell physiology problems, cell pharmacology and for toxicology of heavy elements.
Further insight into biological processes and cellular analysis mainly requires high spatial resolution techniques for intracellular analysis, quantitative data, non-destructive analyses and chemical information. These requirements can be obtained by recent improvements that have been realised in third-generation Synchrotron X-ray sources and in X-ray focusing optics. Highly collimated quasi-monochromatic X-ray beams with tuneable energy and a highly focussed beam with a submicrometre diameter can be used for trace element analysis (X-ray fluorescence) and chemical speciation (XANES) with minimum radiation damage to the sample, short-time analysis and low limit of detection (< ppm).
This work was carried out at ID22. A 14 keV polychromatic "pink"-beam and compound refractive lenses were used, resulting in a beam size of 1 x 10 µm (vertical x horizontal) with a flux of ~ 5.1011 photon/s. Freeze-dried cells were prepared as already published [2].
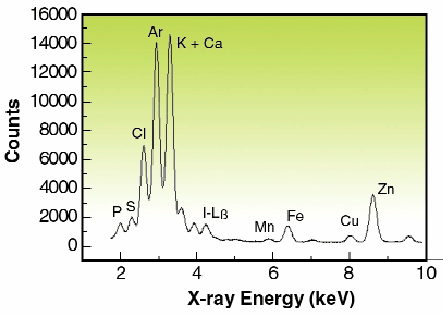 |
Fig. 121: Spectrum from a single human ovarian adenocarcinoma (IGROV1) cell treated with 5 µM of iodo-doxorubicin. It was obtained using a focused 14-keV polychromatic "pink" excitation and 100 sec acquisition time.
|
In Figure 121, the spectrum shows the X-ray peaks of intracellular elements P, S, Cl, Ca, K, Mn, Fe, Cu, Zn and I from drug treatment. Compared to cell observation by light microscopy, potassium maps roughly depict the cell boundaries, particularly the nuclear region. Iodine imaging of cells treated with 5 µM of IDX could be performed in this study and the surface concentrations are reported in Figure 122. SXRF mapping shows an intracellular distribution of trace elements comparable to previous results obtained by micro-PIXE for higher doses of IDX (20 µM) [2]. In particular, the co-localisation of iron and iodine within the cell nucleus is observed (Figure 122, c and d). This was also found using monochromatic excitation (data not shown) but more than 12 hours of mapping were necessary. Using a mean value of freeze-dried cells surface mass (260 µg/cm2) obtained by RBS (Rutherford Backscattering Spectroscopy), maximum concentrations for cells treated with 5 µM IDX were 10340 ppm for potassium, 274 ppm for zinc, 76 ppm for iron and 580 ppm for iodine. These results are in good agreement with previously published data on IGROV1 cells trace elements content [2]. It is important to note that such elemental imaging of cells treated with pharmacological doses of drug cannot be performed by PIXE (Proton induced X-ray emission spectroscopy) because of the low concentration of drug.
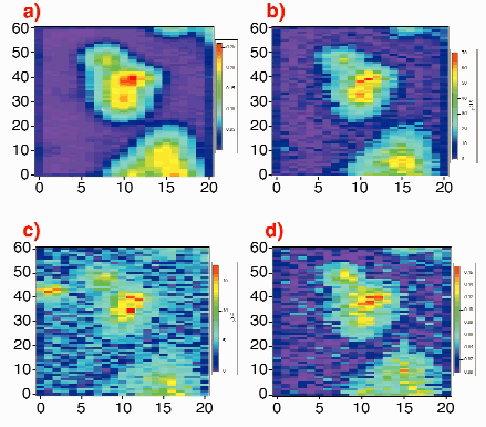 |
Fig. 122: Contour map of a freeze-dried cancer cell treated with 5 µM of iodo-doxorubicin. Cell was mapped with a 14 keV polychromatic "pink" excitation, step size 1 µm x 3 µm (V x H) and 2.5 sec acquisition time/step, scan size: 60 x 60 µm 2, around 2-hours total acquisition time. The surface mass values (colour legend) are expressed in µg/cm 2. a) Potassium distribution (K
|
This experiment opens a new way toward possibilities of mapping intracellular distribution of drugs used at pharmacological doses. SXRF will be highly complementary to laser confocal microscopy or nuclear microprobe analysis, and brings information that is not fully accessible by any other technique. Finally, simultaneous chemical speciation (X-ray absorption spectroscopy) [3] and microanalysis of living cells are exciting perspectives under investigation that will soon be reached with SXRF microprobes.
References
[1] Y. Llabador and P. Moretto, Nuclear microprobe in the Life Science, World Scientific, Singapore (1997).
[2] R. Ortega, P. Moretto, A. Fajac, J. Benard, Y. Llabador, and M. Simonoff, Nucl. Instr. and Meth. B 130, 426 (1997).
[3] R. Ortega, G. Devès, S. Bohic, A. Simionovici, B. Ménez, and M. Bonnin-Mosbah, Nucl. Instr. and Meth. B, to be published.
Principal Publication and Authors
S. Bohic (a), A. Simionovici (a) R. Ortega (b), D Heymann (c), C. Schroer (d) and A. Snigirev (a), to be published.
(a) ESRF
(b) Université Bordeaux 1, Gradignan (France)
(c) Laboratoire de la Physiopatologie de la Résorption Osseuse, Nantes (France)
(d) RWTH Aachen (Germany)



