- Home
- News
- Spotlight on Science
- Filming a catalyst...
Filming a catalyst in action
08-06-2007
Synchrotron light can be used to follow a catalyst under working conditions. X-ray absorption spectroscopy that reveals the oxidation and reduction processes of the catalyst is conventionally performed in steady-state conditions or either temporally or spatially resolved. This spotlight presents an experiment that combines time and space resolution giving deeper insight into the reaction mechanism.
Catalytic reactions are omnipresent in modern chemical synthesis. More than 90% of all chemical products are prepared via at least one catalytic step. Understanding reaction mechanisms and the structure of catalysts is of utmost importance to improve existing catalytic production routes and to find new ones. An important example is the catalytic partial oxidation of methane that can be used for the production of hydrogen and/or synthesis gas. It is regarded as a first step in the so-called "gas-to-liquid technologies" in order to transform natural gas into liquid feedstocks such as methanol. A change in a chemical reaction with time and/or in space is often correlated to a change in state of the catalyst. It is desirable to follow such changes in a spatiotemporal manner on a real catalyst.
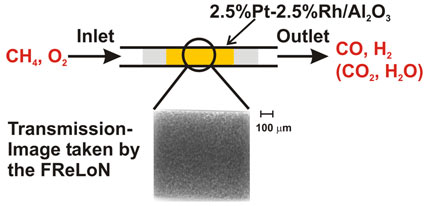
In the present study we have investigated the ignition of the catalytic partial oxidation of methane by time-resolved imaging of the catalytic reactor (quartz capillary of 1 mm diameter; heated by a gas blower). For this purpose, the FReLoN (fast readout low noise) camera developed at ESRF was placed behind the catalytic microreactor (Figure 1) and the structural changes occurring in the catalytic microreactor during ignition of the partial oxidation of methane over a Pt-Rh/Al2O3 catalyst were studied both spatially and with time-resolution (second scale).
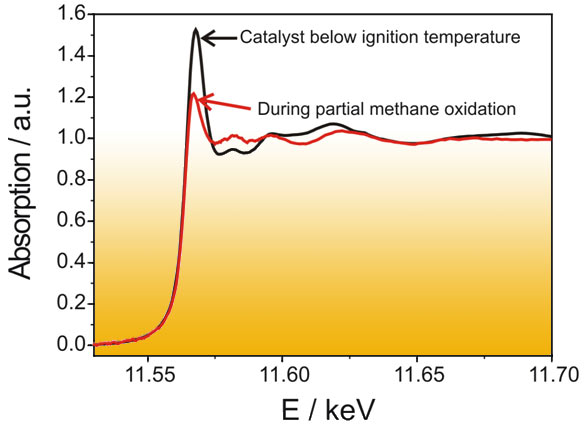
The oxidation state of the noble metals is strongly dependent on the reaction conditions. Below the ignition temperature of methane towards CO and H2 the catalyst is in an oxidized state, whereas above the ignition temperature a reduction of the noble metals occurs. This can be followed by X-ray absorption spectroscopy. Figure 2 shows the XANES spectrum at the Pt L3-edge below and above the ignition temperature. A strong change in absorption occurs at the whiteline of platinum (11.568 keV): its relative height decreases from 1.5 to 1.2 as the oxidized platinum is reduced (cf. Figure 1).
 |
|
Fig. 2: XANES spectra at the Pt L3-edge below and above the ignition temperature of the partial oxidation of methane to hydrogen and carbon monoxide. |
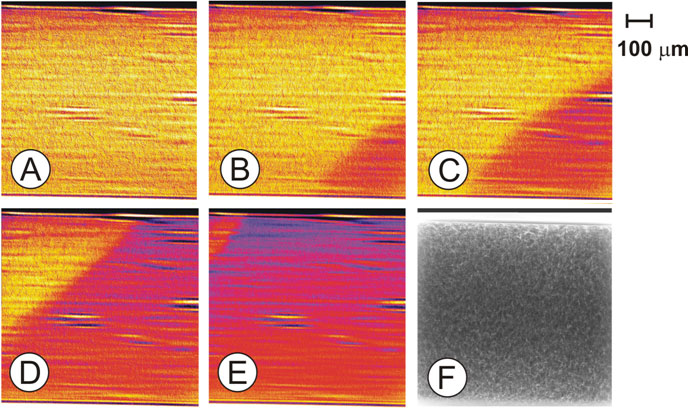
Tuning the energy of the incoming X-rays to the maximum of the whiteline, a decrease of absorption should be visible at those parts of the microreactor, where the platinum constituent is reduced. By collecting X-ray transmission images of the catalytic microreactor with a frame rate of 1s-1 (200 ms exposure time per image), a strong structural change of the catalyst could be found when the ignition of the reaction from methane and oxygen to hydrogen and carbon monoxide started. Selected Figures at time t1 (Pt is still in an oxidized state over the whole catalyst bed) and at time t > t1 are depicted in Figure 3.
 |
|
Fig. 3: X-ray absorption images recorded at the whiteline energy of Pt (Pt L3-edge; 11586 eV) as function of time: (A) t1; (B) t1 + 1 s; (C) t1 + 2 s; (D) t1 + 5 s; (E) t1 + 33 s; (F) transmission image; (A) to (E) were obtained by subtraction of the X-ray absorption image at time t from (F) which was collected at t < t1; t1 is the time of the last image where no gradient was found in the images. |
The figures were obtained by subtraction of the X-ray absorption images at time t from those recorded below the ignition (such as in Figure 3F) to emphasize the difference in absorption and to correct for inhomogeneities in the sample. The full time series of the ignition process is shown in the movie. The film shows the propagation of the frontier between oxidized and reduced catalyst species starting at the outlet towards the inlet of the reactor. The observed reduction of the noble meal occurred exactly at the same time as the chemical reaction ignited (detected by on-line gas analysis using a mass spectrometer). Note that heat was provided from the bottom, which results – on the μm-scale – in an asymmetric profile.
The movement of the front from the outlet towards the inlet can be explained with a total oxidation – reforming mechanism. At first, below the ignition temperature, methane is fully combusted to carbon dioxide and water over the whole length of the catalyst bed. At the very moment, when all oxygen is consumed at the outlet of the reactor, some metallic species form (preferentially at the end of the reactor). They promote the activation of methane and thus lead to the formation of hydrogen and carbon monoxide, which again leads to an enhancement in the formation of hydrogen and carbon monoxide. Thus, the reduction propagates towards the inlet of the reactor, where the total oxidation of methane still occurs over oxidized noble metal particles.
This example demonstrates that it is essential to determine the structure of a catalyst not only in situ but also in a spatiotemporally resolved manner. Thereby, important insight into the mechanism of the catalytic reaction can be gained. In particular, it allows understanding of startup phases of catalytic reactors and reaction mechanisms in more detail.
View the movie:
The movie (Quicktime, 3mb) shows the X-ray absorption at the whiteline energy of the Pt L3-edge (11586 eV) as function of time during the ignition of the partial oxidation of methane over Pt-Rh/Al2O3; the absorption drops from the right (reactor outlet) to the left (reactor inlet), because the noble metals are reduced upon reaction and the reaction front propagates to the left of the microreactor; at t1 = 7s the reaction ignites; brighter colour indicates higher X-ray absorption.
Authors
J.-D. Grunwaldt (a), B. Kimmerle (a), P. Glatzel (c), P. Boye (b), and C.G. Schroer (b)
(a) Institute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich (Switzerland)
(b) Institute of Structural Physics, TU Dresden (Germany)
(c) ESRF