- Home
- News
- General News
- LED lights may be...
LED lights may be bad for Van Gogh paintings
04-01-2013
Dozens of masterpieces are susceptible to light induced darkening of yellow areas.
An international team of scientists has used synchrotron X-rays to better understand why some bright yellow colours in Vincent Van Gogh’s paintings are turning brown with time, while others do not. The research focus was on chrome yellow, a colour favoured by Van Gogh to depict sunshine and light. Several types of this yellow were found to be very sensitive to green and blue light which causes a darkening of the painting. The scientists recommend that museums identify all paintings with this type of chrome yellow and protect them in particular from the increasingly popular LED lights which emit a large amount of blue.
The scientific results were published on 14 November 2012 in the journal Analytical Chemistry.
Previously, the same group of researchers from Italy, Belgium, The Netherlands, France and Germany had discovered that the light yellow tones in Van Gogh’s paintings are especially prone to darkening. This process involves a chemical reduction of chromium, the key element in the chrome yellow pigment. However, they did not understand the origin of this chemical transformation, and why the degradation differed considerably among paintings.
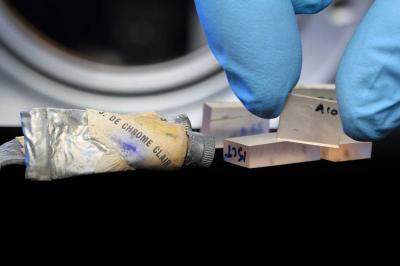 |
|
Several microsamples from art masterpieces, moulded in Plexiglass plates ready for investigation with synchrotron X-rays. The paint tube contains historic yellow chrome paint from the personal collection of M. Cotte. Credit I. Montero/ESRF. |
State-of-the-art X-ray analysis of an extensive series of Van Gogh paintings belonging to three museums in The Netherlands and France allowed the researchers to conclude that Van Gogh did not always use the same type of chrome yellow paint. To confirm these findings, original paints employed by Van Gogh and by Cézanne in the late 19th century were examined. The key experiments, involving speck-sized paint fragments of the Van Gogh paintings, were performed at two synchrotron facilities, the ESRF in Grenoble (France) and DESY in Hamburg (Germany) as well as in cultural heritage laboratories in Italy and The Netherlands.
|
|
|
A sample of chrome yellow paint with pigment sensitive to blue light after several hundred hours of artificial aging with light of different nature: UV-Vis = wide spectrum of visible and ultraviolet light; UV = ultraviolet light only; blue and red light only. Credit: University of Antwerp, Department of Chemistry. |
Next to regular lead chromate, also known as "middle yellow", which is quite stable chemically, Van Gogh also liked to use "lemon yellow" and "primrose yellow".
Some of the 'non-regular' types turned out to be fairly reactive under light as they have a different crystal structure and/or a higher sulfate content. The more sulphur present in a chrome yellow pigment, the more unstable it becomes. In laboratory tests with different samples of chrome yellow paint, those containing the unstable varieties turned brownish/olive green after just a few days of exposure to green-blue light. Regular chrome yellow maintained its colour after exposure to green blue light and also when irradiated with more damaging UV light and when exposed over longer periods.
"We have found the unstable forms of chrome yellow in several well-known paintings, such as 'Portrait of Gauguin' and the famous 'Vase with Sunflowers' of the Van Gogh museum in Amsterdam", says Letizia Monico, a Chemistry PhD student in Perugia (Italy) and Antwerp (Belgium) and key investigator.
"As we are aware that both stable and unstable forms of chrome yellow paint were used not only by Vincent Van Gogh but also by Paul Cézanne, we advise museums that own such masterpieces to ascertain whether the unstable forms of chrome yellow were used and, if needed, to tailor their lighting systems accordingly", summarises Koen Janssens from the University of Antwerp, who coordinated the team.
Costanza Miliani (CNR, Italy) emphasised that "we were surprised to note that already under conditions of illumination currently considered safe, some of our test samples started changing colour quite quickly".
Bruno Brunetti (Univ. of Perugia) adds, "We could show it is possible to establish which type of chrome yellow is present in a painting by means of relatively simple equipment based on reflection of infra-red light, that may be used in the museum galleries themselves."
Ella Hendriks, Head of Conservation at the Van Gogh Museum in Amsterdam concludes, "Studies like these are very important to make museum curators aware that even under ambient light conditions, the degradation of some sensitive materials proceeds continuously. Museums should carefully consider the potential impact of, for example, lights such as the new LED-based systems that are now being installed in collections."
Top image: “Portrait of Gauguin” by Vincent Van Gogh (1888). Credit: Van Gogh Museum Amsterdam.





