- Home
- News
- General News
- Repulsion brings...
Repulsion brings organic molecules into line
11-03-2009
The idea of conductive plastic may seem contradictory, but “organic electronics” have the potential to revolutionise the host of semi-conductor devices that make up the components of modern life. LEDs, transistors and photovoltaic cells made with organic semiconductors could be lighter, more flexible, easier to process and most importantly, much cheaper. However, even though organic electronics are already commercially available in some fields, they still lag behind in efficiency compared to their inorganic counterparts. One important aspect which still has to be improved is the molecular order within the organic thin films. German researchers have found a novel interaction whereby organic molecules self-assemble into well-ordered thin films, and have developed a new theory to explain it.
The most commonly used inorganic semiconductor, silicon, is very well ordered - all the atoms occupy a regular pattern, and it is relatively easy to make the material highly conductive. In contrast, organic semiconductors are made of complex molecules, which may not take on any overall regular order. Electrons travel through this organic material by “hopping” from molecule to molecule. In general this works better when the device is well ordered. Therefore molecular order is an important issue, and can be best achieved when the very first layer of molecules in the active region already shows good long range order. Normally, when molecules are sprayed onto a surface, they tend to attract each other and gather together in patches or ‘islands’ which, on a nanometre- or micrometre- scale, gives a lumpy, irregular initial layer – and therefore everything built on top is patchy too.
Now researchers from the University of Würzburg and the Forschungszentren Jülich and Karlsruhe, all in Germany, have found a mechanism which might bring the molecules into line on a much larger length scale than before. They deposited molecules of the organic compound Sn-phthalocyanine onto a flat plane of silver. After landing, the molecules are still able to move across the surface and they show a remarkable behaviour: they repel each other, maximizing the distance to their neighbours, a strange behaviour that has not been seen before for molecules like this. What’s more, upon cooling to approximately -100°C they form another regular arrangement, in which they get in close contact. The repulsion seems to be “switched off”, just by changing the temperature. In the low-temperature structure the flat molecules arrange themselves neatly on the silver substrate with their ‘nose’ bobbles (the Sn-atom) alternately pointing up and down. Then, as the temperature increases, or with the addition of more molecules, they cannot maintain this energy-minimizing situation and the repulsive force becomes dominant, leading them to lose this regimented appearance and just move as far apart as possible. As coverage increases, the symmetrical molecules are forced closer and closer together, prevented from overlapping by the repulsion between them - until they give a perfectly tiled surface.
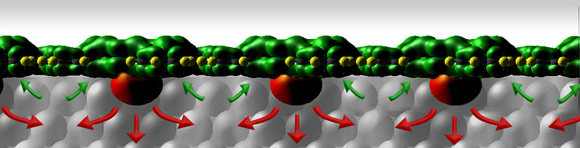
Further observations via X-ray standing waves performed at the ESRF showed that in this final ordered state, all the molecules have their ‘noses’ pointing down. Since the ‘nose’ is formed by the electrons located close to the Sn-atom, the team’s first theory was that this was giving the molecules a slight negative charge at the bottom and a positive charge at the top, with the electric charges causing repulsion. The scientists decided to repeat the experiment using a “nose-less” molecule: Cu-phthalocyanine. But even though this molecule is perfectly flat and lacks the asymmetric electron bulge, they obtained almost exactly the same results.
So the team had to come up with a new theory to explain these two results in terms of electron sharing between the silver substrate and the molecules. Their conclusion was that the free electrons from the central metal atom of the phthalocyanine were pushing their way into the substrate, while electrons were donated from other surface atoms to fill the holes that were left in the molecule. As each molecule competes for their share of silver electrons to swap, they move apart to take advantage of all the resources available, creating the uniform layer observed. With this smooth start, the addition of more molecular layers resulted in a regular, very well ordered, semi-conducting organic crystal. Such an ordered environment allows much better electron transport, and if these characteristics can be preserved in an industrial context, organic electronic devices might see much improvement. Maybe we’ll finally have our electronic paper, spray-on home cinema screens and parasols made of solar panels. If not, we have at least found an interesting, unexpected piece of physics and understood the world a little bit better.
|
A side view of a row of Sn-phthalocyanine molecules on a silver crystal face. The red arrows indicate the movement of electrons donated from the phthalocyanine 'nose' into the silver: the green arrows indicate the passage of electrons from the substrate back into the receptive areas of the molecules. The net effect of the electron transfer is to push the molecules apart. |
Reference:
C. Stadler et al., Nature Physics, January 2009. DOI: 10.1038/NPHYS1176
Top image: A single Sn-phthalocyanine molecule on a silver crystal face. The locations of electrons are shown in red (the 'nose'), and the areas into which the molecule can accept electrons are coloured green. The molecule is shown in the 'nose-up' position.