- Home
- News
- General News
- Change of paradigm...
Change of paradigm for skeletal muscle regulation
10-11-2015
Scientists from Italy and the United Kingdom, in collaboration with the ESRF, have demonstrated a new regulation mechanism which provides a novel explanation for the dynamic and energetic properties of skeletal muscle. These findings offer a promising approach for new investigations on the regulation of cardiac muscle and new therapeutic opportunities.
An international team of scientists from the University of Florence (Italy) and King’s College London (United Kingdom), in collaboration with the ESRF, the European Synchrotron, Grenoble (France), demonstrated a new regulation mechanism for skeletal muscle.
Contraction of striated muscles (skeletal and cardiac muscles) was thought to be controlled by a calcium-dependent structural change in the actin-containing thin filaments that permits the binding of myosin motors from the neighbouring thick filaments to drive filament sliding. By synchrotron X-ray diffraction from single skeletal muscle cells, the scientists have shown that muscle contraction is actually controlled by two switches. They demonstrated that, although the well-known thin-filament mechanism is sufficient for regulation of muscle shortening against low load, force generation against high load requires a second permissive step linked to a change in the structure of the thick filament. This concept of the thick filament as a regulatory mechano-sensor provides a novel explanation for the dynamic and energetic properties of skeletal muscle. A similar mechanism is likely to operate in the heart, another striated muscle. This fundamental result, published in Nature, therefore offers a promising approach for new investigations on the regulation of the cardiac muscle and new therapeutic opportunities.
 |
 |
|
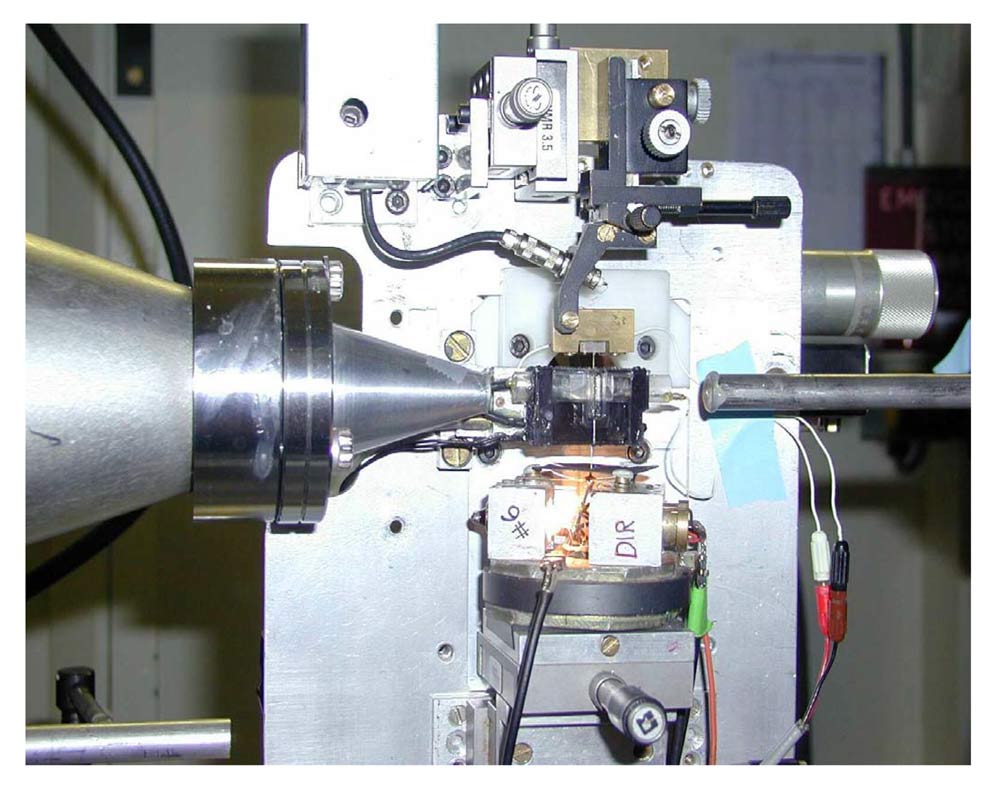
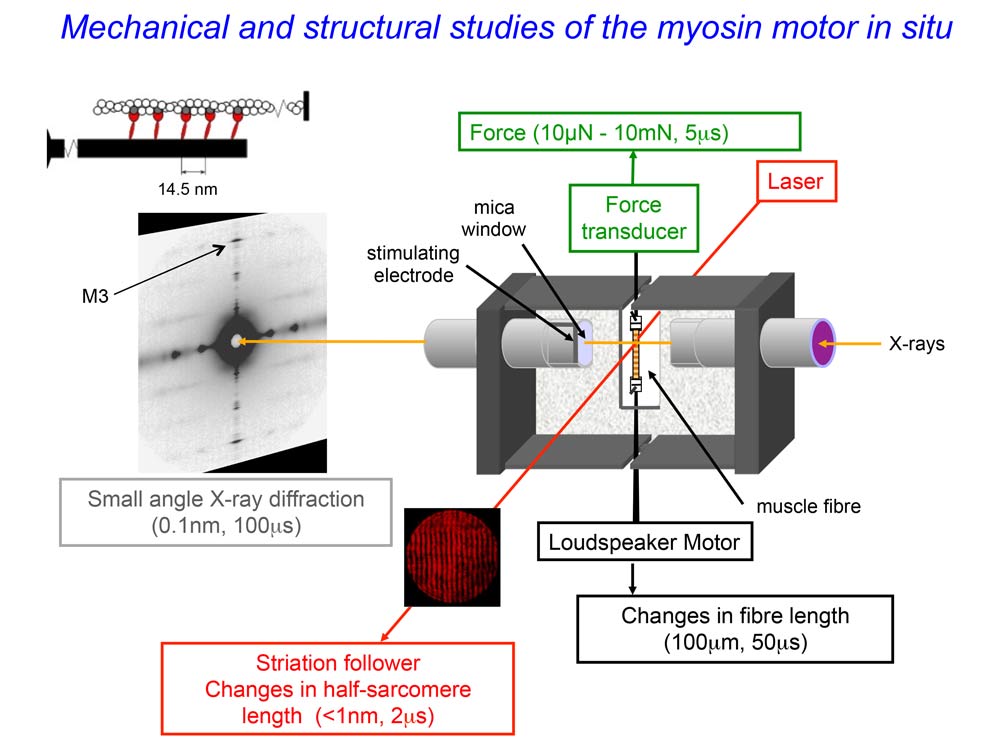
Picture and schema of the experimental set-up with the muscle cell mounted vertically in a trough between the loudspeaker motor (bottom) and the force transducer (top). The small-angle X-ray diffraction pattern is recorded by a FReLoN detector. Credit: Vincenzo Lombardi. |
|
Striated muscles (skeletal and cardiac muscles) contract by the relative sliding motion of two sets of overlapping filaments containing the proteins actin and myosin. According to the textbook view, contraction is triggered by the rise of the concentration of calcium ions that follows excitation of the muscle cell by a motor nerve. Binding of calcium ions to regulatory proteins in the thin actin filament releases them from their inhibitory action, allowing myosin motors from the thick myosin filament to attach and pull the actin filament in the shortening direction.
An international team of scientists from Italy, the UK and France has now demonstrated a new regulatory mechanism. For this discovery, they used small-angle X-ray diffraction from the intense light source of the ESRF to observe at the molecular scale how muscle proteins change structure inside a contracting skeletal muscle cell. This new regulatory mechanism allows the number of myosin motors to be adapted to the force developed by the contraction. When the load is low, the action of a small fraction of the motors drives muscle shortening at high velocity within a few milliseconds of the excitation, when calcium ions have activated the actin filament. When the load is high, the mechano-sensing property of the myosin filament is responsible for recruitment of the much larger fraction of motors required to generate a high force.
|
|
| A few members of the research team on the ESRF's ID02 beamline. From left to right: N. Theyencheri (ESRF), M. Linari, G. Piazzesi, V. Lombardi, M. Reconditi (University of Florence), and G. Steinen (University of Amsterdam, not involved in this research project). Credit: ESRF/C. Argoud |
What is the significance of this new mechanism of regulation?
Malcolm Irving, from King’s College London and co-author of the paper, says: “Conventionally, contraction of the muscles that move the skeleton was thought to be controlled through the actin filament, which is the track along which the myosin motors from the myosin filaments move, rather than through the motors themselves. However that view became increasingly difficult to reconcile with emerging evidence that the myosin motors in resting muscle are immobilised or switched OFF and cannot interact with actin. How could such a switched OFF myosin motor sense the state of the actin filament at a distance? The new results provide an answer: most of the myosin motors in resting muscle are switched OFF, and this is probably important for minimising the resting metabolism of the muscle cells, but a small fraction of motors can bind to actin and sense its regulatory state. Not only can this small fraction of motors drive unloaded shortening with high metabolic efficiency, but they also have a signalling function: when the external load on the muscle is high, they stretch the myosin filaments, and that stretch provides the signal to unlock the majority of motors only when they are needed for contraction against a high load. “
New perspectives for the regulation of cardiac muscle
As thick filament structure and protein composition are essentially the same in heart and skeletal muscle, thick filament mechano-sensing may also be a fundamental component of the regulation of contractility in the heart. This discovery opens the possibility of new approaches for therapeutic control of cardiac output.
Vincenzo Lombardi, from University of Florence and co-author, says: “Our results show that muscle contraction is controlled by two switches: the actin filament switch conveys the ON signal from the central nervous system, and the myosin filament switch adapts the response of the muscle to the external load, ensuring that its efficiency is maximised. However, this is not the only way the myosin motor regulates its activity. Other contractile systems, like smooth muscle, which do not require a fast response, use an intra-molecular mechanism of regulation that consists of an increase in the level of phosphorylation of the regulatory portion of the motor itself. The discovery that in skeletal muscle the myosin filament acts as a mechano-sensor that adapts the number of motors to the external load gives a paradigmatic example of how the evolution of the motor system in this case has optimised performance and efficiency and suggests a promising approach for new investigations on the regulation of cardiac muscle”.
“This work again illustrates the uniqueness of small-angle X-ray diffraction based muscle interferometry. Presently we are striving to optimise this technique for cardiac muscle. This method will become even more powerful for muscle research after the new ESRF upgrade programme, the ESRF-EBS project, which will provide us with an extremely brilliant source ”, adds Theyencheri Narayanan scientist in charge of ESRF's ID02 beamline, and co-author.
Reference:
Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments
Marco Linari, Elisabetta Brunello, Massimo Reconditi, Luca Fusi, Marco Caremani, Theyencheri Narayanan, Gabriella Piazzesi, Vincenzo Lombardi and Malcolm Irving - Nature - 10.1038/nature15727
Top image: 3D rendering of a skeletal muscle fibre. Credit: Wikiversity Journal of Medicine.




