- Home
- News
- General News
- A photoenzyme allows...
A photoenzyme allows microalgae to produce hydrocarbons
01-09-2017
An international team has discovered an enzyme which allows microalgae to convert some of their fatty acids into hydrocarbons using light. This enzyme, which has been named “FAP” (Fatty Acid Photodecarboxylase), is of an extremely rare type, as only four enzymes powered by light have been identified to date. This discovery is relevant in the quest of sustainable energy production.
Chlorella is a single-cell freshwater alga belonging to a type of microalgae which are cultivated on an industrial scale and have potential for the production of energy-rich molecules. Researchers at the CEA (French Atomic Energy Commission), the CNRS (French National Centre for Scientific Research), the ESRF, INSERM (French National Institute of Health and Medical Research) and the Universities of Aix-Marseille, Grenoble Alps and Paris-Sud have discovered an enzyme in this algae which allows it to convert some of its constituent fatty acids into hydrocarbons using light energy only. The researchers believe that this is a major advance in the identification of these kind of biological mechanisms and opens up a new option for the synthesis of hydrocarbons by micro-organisms on an industrial scale.
In this study, researchers from the Institute of Biosciences and Biotechnologies of Aix-Marseille (CEA/CNRS/University of Aix-Marseille), identify this key enzyme for the synthesis of hydrocarbons, by following its activity and determining a list of potential candidate proteins using a proteomic analysis conducted at the laboratory of Large scale biology (CEA/INSERM/University of Grenoble Alps). The expression in the bacterium E. coli of the encoding gene for the main candidate protein has shown evidence of the production of hydrocarbons, thereby demonstrating that this enzyme is both necessary and sufficient for hydrocarbon synthesis. Characteristic analysis of the pure enzyme revealed that it is capable of splitting a fatty acid into a hydrocarbon molecule and a CO2 molecule, and that this activity requires light.
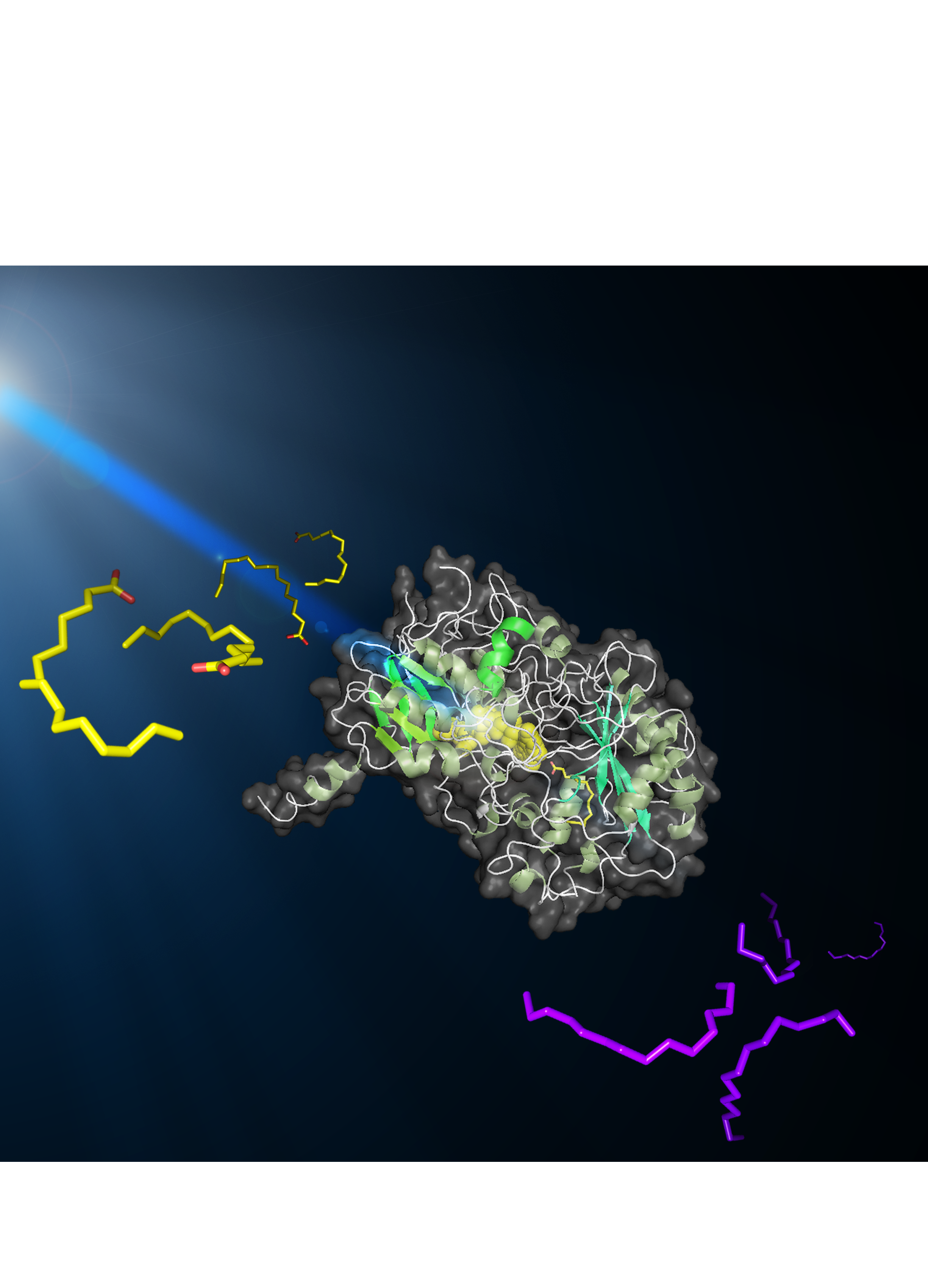 |
|
Image of the FAP molecule while it converts the fatty acids in hydrocarbons under the blue light. The FAP is in the middle. On its left, the fatty acid molecules are yellow; on the right in purple, the hydrocarbon molecules. Credits: Caroline EPLE. |
Researchers have also shown that a co-factor (a non-protein molecule, which is associated with the enzyme and is necessary for its activity) present in the enzyme permits the capture of blue light. The three-dimensional structure of the enzyme (Figure 2), determined by X-ray diffraction analysis conducted on the fully-automated “MASSIF-1” beamline of the European Synchrotron Radiation Facility (ESRF, Grenoble), and kinetic absorption spectroscopy analyses done at the Institute of Integrative Cell Biology (CEA/CNRS/University of Paris-Sud), have led to the formulation of a model for the mechanism of the enzyme. The fatty acid is positioned in a hydrophobic tunnel, at the end of which the co-factor is located. The latter, upon excitation by blue light, removes an electron from the carboxyl group of the fatty acid, resulting in spontaneous decarboxylation to form a hydrocarbon molecule.
The discovery of this enzyme is of major and fundamental interest, given that only four biocatalysts capable of exploiting light energy (photoenzymes) are known. These are an enzyme for the repair of DNA, an enzyme for chlorophyll synthesis, and the reaction centres for the two photosystems that permit photosynthesis in plants and algae. FAP is at least ten times faster than the best-known enzyme for hydrocarbon synthesis, and uses light, thus potentially providing a highly effective biotechnological tool for the synthesis of hydrocarbons, either by the in vitro conversion of oils, or by the in vivo conversion of the membrane fatty acids of bacteria, yeasts or, ideally, microalgae.
Top image: Chlorella. Credits: ja:User:NEON



