- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- X-ray nanoprobe
- Biochemistry of the malaria parasite by X-ray microscopy
Biochemistry of the malaria parasite by X-ray microscopy
The distribution and concentration of chemical elements associated with heme detoxification were mapped using combined hard X-ray cryo fluorescence microscopy and soft X-ray cryo tomography study of rapidly frozen malaria parasites. These studies aim to discover ways to sabotage the detoxification of heme accumulated in the malaria parasite during its consumption of hemoglobin.
Malaria in humans is caused by the parasite Plasmodium falciparum. The parasite, injected by an Anopheles mosquito, travels the liver where it multiplies, producing thousands of new parasites. The next generation of parasites enters the blood stream and invades red blood cells, where they feed on hemoglobin (Hgb) in order to grow and multiply. After creating up to 20 new parasites, the red blood cell bursts, releasing the daughter parasites ready for new invasions. This life cycle leads to an exponential growth of the infected RBCs that may cause the death of the human host.
The aim of the research described here was to identify mechanisms critical for the parasite’s survival, thereby providing an intelligent basis for the development of drugs to interrupt the exponential growth of the infection. One such mechanism is the detoxification of heme, an iron-containing molecule that is released into the parasite upon hemoglobin degradation. The parasite neutralises heme by turning it into harmless hemozoin crystals that are built from heme dimers. Therefore, heme dimerisation and the subsequent crystallisation of the heme dimers are obvious goals for detailed experimental investigation.
Advanced X-ray methods have been used to investigate the parasite-infected red blood cells [1-3]. In the present study, a combination of hard X-ray cryo fluorescence microscopy at beamline ID16A and soft X-ray cryo tomography at MISTRAL/ALBA was used. The infected red blood cell is immobilised by rapid cooling to 80 K, leaving the intracellular water in an amorphous, so-called vitrified state. The cryo-temperature also essentially eliminates radiation damage. The tomography is based on absorption contrast just like CT scanning at a hospital, but the sample diameter is that of a single cell, about 5 micrometres in diameter. The X-ray energy was chosen to be between the absorption edges of carbon and oxygen (510 eV), rendering the vitreous ice essentially transparent, while the carbon content remained highly absorbing. In this way, the structure of the infected red blood cell can be mapped in three dimensions (3D) while radiation damage is avoided.
X-ray fluorescence mapping of the sample was carried out using a hard X-ray beam with a diameter of about 30 nm, permitting a sample to be scanned and the fluorescent signal registered from chemical elements ranging from sulfur (S) to iron (Fe). Fe is bound to heme and is abundant in Hgb and in the hemozoin crystals, but the concentration of Fe atoms in hemozoin is much higher than in Hgb. Furthermore, the fluorescence signal from Fe in Hgb is accompanied by the fluorescence from S since there are 12 S atoms and 4 Fe atoms in a single Hgb molecule. Thus it is possible to disentangle whether heme is bound in hemozoin crystals or in Hgb. By overlaying the 3D parasite structure and the X-ray fluorescence maps, the distribution and concentration of chemical elements can be measured within various compartments of the parasite (see Figure 72). Notably the potassium is relocalised to the parasite in an infected red blood cell.
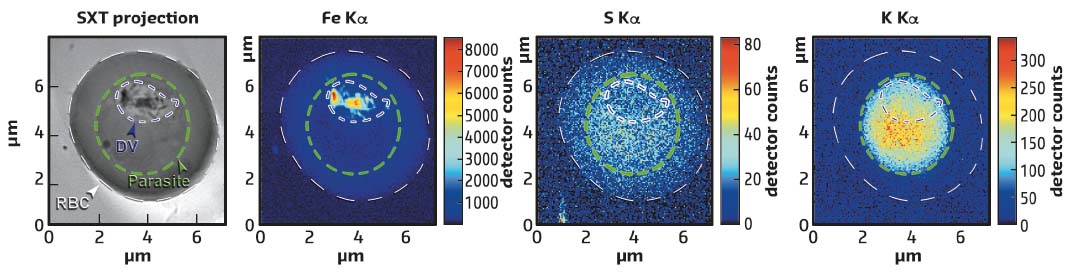 |
|
Fig. 72: Red blood cell (RBC) infected with P. falciparum studied by soft X-ray tomography (SXT) visualising the 3D structure of the parasite and X-ray fluorescence producing maps of iron, sulfur and potassium. The infected cell, the parasite and the digestive vacuole (DV) are outlined with white, green and blue dashed lines, respectively. |
The liberation of heme monomers from Hgb, their subsequent dimerisation and, finally, the crystallisation of the heme dimers, all take place in a parasite organelle called the digestive vacuole, (DV). Previous measurements of parasites that have spent between 30 and 45 hours in red blood cells have shown that there is considerable non-crystalline heme in the DV in the form of Hgb, and that in vivo heme crystallisation occurs at a rate of about 104 per second. It has been suggested that the dimerisation needed for heme crystallisation is furnished by a protein called heme detoxification protein also with a rate of about 104 per sec, which is determined by in vitro experiments. This has led to the proposal of an assembly-line model where heme liberation, dimerisation and crystallisation take place at the same rate regulated by a feedback mechanism.
Future research will include a systematic study of the evolution of heme liberation, dimerisation and detoxification with the parasite’s age.
Principal publication and authors
Biochemistry of malaria parasite infected red blood cells by X-ray microscopy, S. Kapishnikov (a), L. Leiserowitz (b), Y. Yang (c), P. Cloetens (c), E. Pereiro (d), F. Awamu Ndonglack (e), K. Matuschewski (e) and J. Als-Nielsen (a), Sci Rep. 7, 802 (2017); doi: 10.1038/s41598-017-00921-2.
(a) Niels Bohr Institute, University of Copenhagen (Denmark)
(b) Department of Materials and Interfaces, Weizmann Institute of Science, Rehovot (Israel)
(c) ESRF
(d) ALBA Synchrotron Light Source, Cerdanyola del Valles, Barcelona (Spain)
(e) Molecular Parasitology, Institute of Biology, Humboldt University, Berlin (Germany)
References
[1] S. Kapishnikov et al., PNAS 109, 11184-11187 (2012).
[2] S. Kapishnikov et al., PNAS 109, 11188-11193 (2012).
[3] S. Kapishnikov et al., Sci Rep. 7, 7610 (2017).



