- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Structural biology
- Zinc fastens two amyloid-β peptide fragments forming stable dimer
Zinc fastens two amyloid-β peptide fragments forming stable dimer
Binding of metal ions to amyloid-β is an initial step of the peptide transition to pathogenic forms toxic for neurons. To understand the molecular mechanisms of zinc-mediated amyloid-β aggregation, amyloid-β peptide carrying a pathogenic Taiwanese mutation was studied. Spectroscopy showed that zinc ions induce formation of a stable homodimer with a novel binuclear zinc interaction fold.
Zinc ions are crucially involved in pathogenesis of Alzheimer’s Disease (AD). This neurodegenerative disorder is characterised by extracellular accumulation of amyloid-β peptide (Aβ) in proteinaceous inclusions that have characteristic supramolecular structure and are abnormally enriched by Zn, Cu and Fe ions. Aβ interacts with metal ions through its metal-binding domain, which is located in its N-terminal region (residues 1-16). Substitutions and modifications of amino acid residues in this domain critically affect the properties of A-β, facilitating dimerisation, oligomerisation and the formation of insoluble aggregates. These processes are considered essential for the initiation of Alzheimer’s disease.
Aβ carrying a Taiwanese mutation (D7H) stands out among all isoforms of Aβ because of the enhanced susceptibility of the peptide to the effect of Zn2+ or Cu2+ ions promoting oligomerisation [1]. Based on the molecular mechanism of formation of zinc-bonded dimers of different Aβ isoforms [2], it was hypothesised that zinc-dependent aggregation of the metal-binding domain of Aβ with Taiwanese mutation is determined by the properties of the fragment containing residues 1-10 (D7H-Aβ(1-10)), where the relevant amino acid substitution is located.
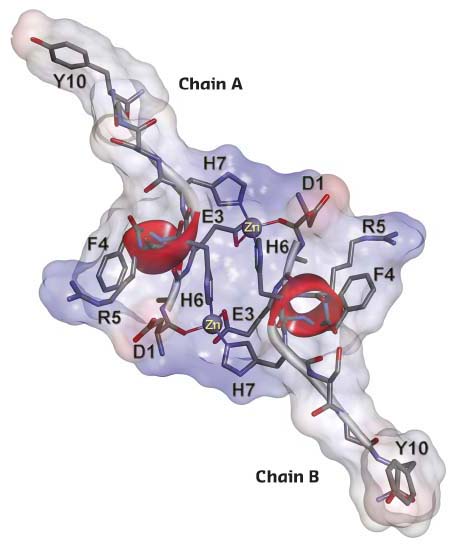
In this study, using NMR spectroscopy, mass spectrometry, EXAFS spectroscopy on BM31 and isothermal titration calorimetry (ITC), the behaviour of this peptide fragment in the presence of zinc ions was examined. The formation of a homodimer with a unique protein fold interlocked by two zinc ions (Figure 19) was observed by NMR. Most of the known examples of metal-directed peptide self-assembly are based on the formation of one or several mononuclear coordination sites. Examples of the formation of a binuclear coordination site with the participation of two zinc ions are still unknown, except when cysteine thiol groups are involved in the binding of zinc atoms.
 |
|
Fig. 19: NMR solution structure of the complex of D7H-Aβ(1-10) with Zn2+sup>. The representative conformer of D7H-Aβ(1-10) zinc-bound complex is shown. The solvent-accessible molecular surface is calculated for all atoms and coloured according to the interpolated charge distribution (red negative, blue positive, grey neutral). |
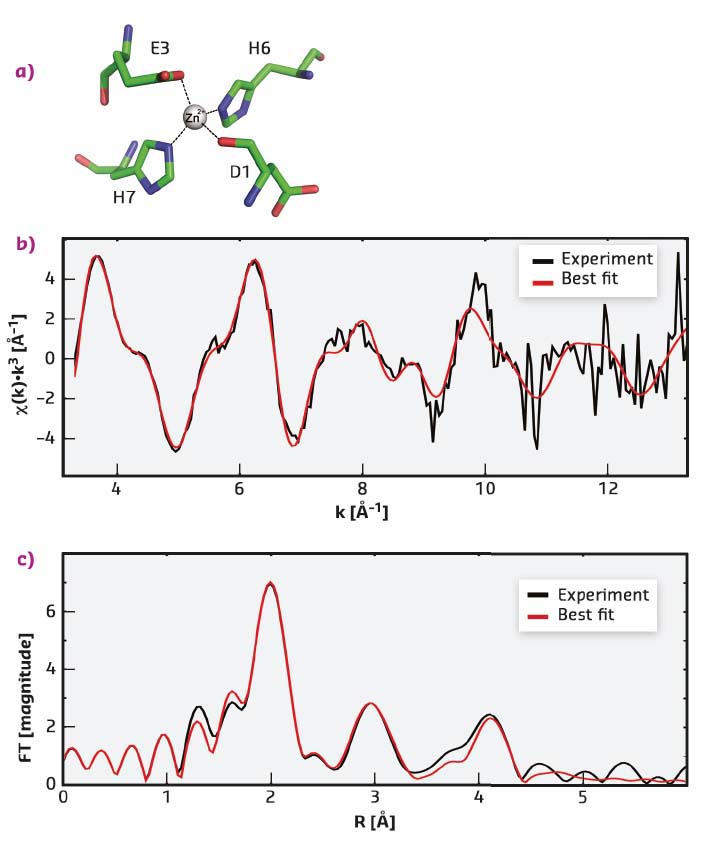
It has been shown here for the first time that a site, with a novel topology for the coordination sphere of zinc ions, is formed upon the interaction of zinc with a relatively short fragment of A-β peptide carrying Taiwanese mutation D7H. The NMR structure determined was validated using extended X-ray absorption fine structure (EXAFS) spectroscopy, which provides information on the geometry of the metal ion chelating environment. EXAFS data give access to the nature, number, and distance of the coordinating atoms. The experimental EXAFS spectrum of the complex of D7H-Aβ(1-10) with zinc fits well to the spectrum, calculated on the basis of the coordinates of atoms determined by NMR (Figure 20). The scattering pathways for the atoms of the first coordination shell (D1 O, E3 Oε1, H6 Nε2 and H7 Nε2) reproduce the most intense peak at ~2 Å of the Fourier-transformed EXAFS spectrum (Figure 20c), while atoms of the histidine imidazole ring make a major contribution to the peaks at ~3 Å and ~4 Å.
 |
|
Fig. 20: Experimental and best-fitted simulated EXAFS spectra of Zn2+ in complex with D7H-Aβ(1-10). a) The Zn2+ coordination used for calculations. b) k3-weighted experimental (black) and least-squares fitted (red) EXAFS spectra. c) the corresponding phase-shift-corrected Fourier transforms. |
Principal publication and authors
A Binuclear Zinc Interaction Fold Discovered in the Homodimer of Alzheimer’s Amyloid-β Fragment with Taiwanese Mutation D7H, V.I. Polshakov (a,b), A.B. Mantsyzov (b), S.A. Kozin (a), A.A. Adzhubei (a), S.S. Zhokhov (b), W. Van Beek (c), A.A. Kulikova (a), M.I. Indeykina (d), V.A. Mitkevich (a) and A.A. Makarov (a), Angew. Chem. Int. Ed. 56, 11734-11739 (2017); doi: 10.1002/anie.201704615.
(a) Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow (Russia)
(b) Faculty of Fundamental Medicine, M.V. Lomonosov Moscow State University, Moscow (Russia)
(c) SNBL, ESRF
(d) Emanuel Institute for Biochemical Physics, Russian Academy of Sciences, Moscow (Russia)
References
1] W.-T. Chen et al., PLoS One 7, e35807 (2012). [2] A.N. Istrate et al., Sci. Rep. 6, 21734 (2016).



