- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Matter at extremes
- Localisation and speciation of impurities in deactivated petrochemical heterogeneous catalysts
Localisation and speciation of impurities in deactivated petrochemical heterogeneous catalysts
Fluid catalytic cracking (FCC) catalysts are key to the production of petrol from crude oil. Here, the iron species in deactivated catalysts were characterised using energy-dispersive XAS tomography.
Fluid catalytic cracking is a century-old chemical conversion process that to this day provides the majority of the world’s petrol. It employs porous composites of zeolite and clay as catalyst. Owing to the harsh reaction environments and feedstock impurities, the catalysts deactivate over time. This deactivation necessitates their continuous fractional replacement with major refineries requiring up to 50 tons of fresh catalyst per day.
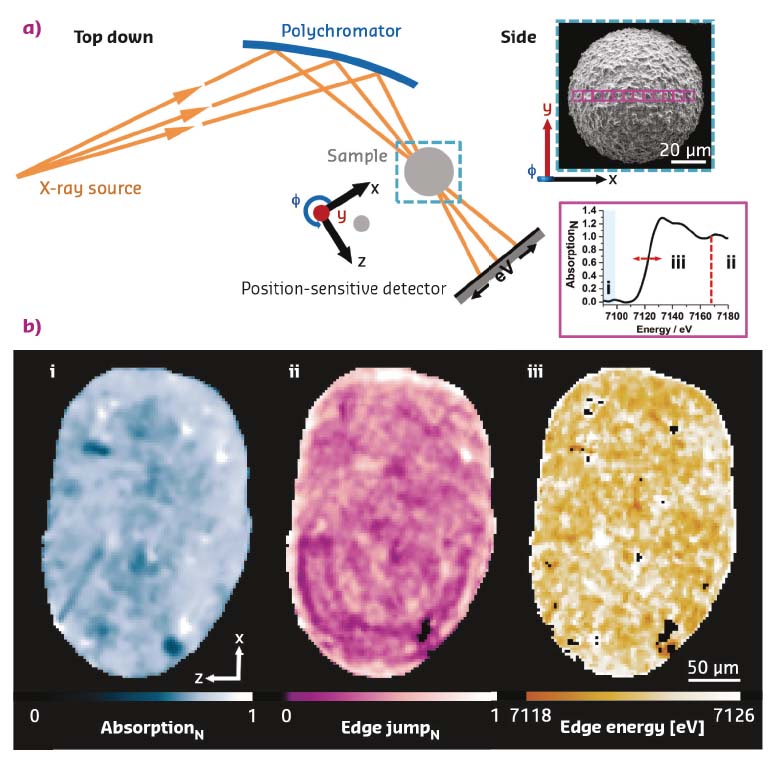
Using a recently developed variant of energy-dispersive X-ray absorption spectroscopy at beamline ID24 [1], the distribution and speciation of feedstock-introduced iron impurities have been visualised in a deactivated fluid catalytic cracking catalyst. Applied to the Fe K-edge, two populations of iron impurities, with specific localisation, were identified. While ferrous iron is found in isolated areas of increased porosity, suggestive of iron particulates, ferric iron is found dominantly on the particle exterior. The latter impurities are preferentially located in the outer dense part of the particle, and suggested to contribute to the formation of an isolating amorphous silica alumina (ASA) envelope that reduces the flux to the active sites in the catalyst interior, Figure 43.
 |
|
Fig. 43: Scanning energy-dispersive tomographic X-ray absorption spectroscopy of a deactivated fluid catalytic cracking (FCC) catalyst. a) Schematic of the experimental setup. Using a polychromatic fan and a position sensitive detector, a set of tomographic projections (φ) were collected. Each consisted of a line scan, step size 2 µm, central to the particle. The electron micrograph shown in the inset is representative of a typical FCC particle (probed regions indicated). Analysis of the acquired projections as a function of position and rotation allowed the construction of one sinogram per energy that can be used to reconstruct a spectro-tomographic volume in which each voxel contains a full Fe-K edge XANES spectrum (7080-7180 eV, in the present case). b) Orthoslices, slices central to the particle, each highlighting selected features in the XAS data, namely (i) absorption below the edge, indicative of density of the material, (ii) edge jump magnitude normalised with respect to the presented field of view, showing the local iron concentration, and (iii) Fe K-edge energy, revealing the iron speciation. |
Considering the operational temperature range and measured impurity speciation in this outer envelope, this suggests that feedstock-introduced iron impurities contribute to the amorphisation of zeolites close to the particle exterior. Such amorphisation could lead to the formation of the apparently non-permeable ASA envelope. Both factors could contribute to the deactivation of the catalyst particle by reducing the overall number of active sites and the ease of access of the remaining active sites. Based on these observations, new conditions for catalyst regeneration and redesign are currently being explored.
The energy-dispersive tomographic absorption spectroscopy variant, currently unique to ID24, sets itself apart from existing XAS tomography techniques because a full XANES spectrum with an energy range of several hundred eV can be acquired in a single measurement. The method is less sensitive to temporal beam and sample instabilities compared to energy-scanning variants. Furthermore, given that a continuous XANES spectrum per voxel is retrieved, with sub-eV resolution, this method is perfectly suitable for the examination of unknown/unexpected chemical species, i.e. non-reliant on tabulated elemental standards. In summary, the energy-dispersive tomographic XAS variant used in this research is an attractive alternative to existing techniques for the examination of extended specimens of unknown elemental speciation with potential applications in heterogeneous catalysis, energy materials, planetary and cultural heritage research.
Principal publication and authors
Localization and speciation of iron impurities within a fluid catalytic cracking catalyst, J. Ihli (a), D. Ferreira Sanchez (a), R.R. Jacob (a), V. Cuartero (b), O. Mathon (b), F. Krumeich (c), C. Borca (a), T. Huthwelker (a), W.-C. Cheng (d), Y.-Y. Shu (d), S. Pascarelli (b), D. Grolimund (a), A. Menzel (a) and J.A. van Bokhoven (a,c), Angew. Chem. Int. Ed. 56, 14031-14035 (2017); doi: 10.1002/anie.201707154.
(a) Paul Scherrer Institut, Villigen (Switzerland)
(b) ESRF
(c) ETHzürich, Institute for Chemical and Bioengineering, Zurich (Switzerland)
(d) W.R. Grace, Refining Technologies, Columbia (USA)
References
[1] D. Sanchez Ferreira et al., Sci. Reports 7, 16453 (2017)



