- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Complex systems and biomedical sciences
- Revealing the molecular details of sensor-histidine-kinase regulation by time-resolved X-ray solution scattering
Revealing the molecular details of sensor-histidine-kinase regulation by time-resolved X-ray solution scattering
Time-resolved X-ray solution scattering reveals the conformational changes that occur in the light-responsive histidine kinase YF1 upon blue-light absorption. These experiments provide novel insight into the regulation of the widespread sensor histidine kinases.
Sensor histidine kinases are part of the so-called two-component signalling pathways, and they are the predominant way for bacteria to gather information about their surroundings. The photoreceptor YF1 serves as a paradigm for this protein family. The homodimeric YF1 receptor consists of a blue-light-sensing light-oxygen-voltage (LOV) photosensor, an α-helical coiled-coil linker, and a histidine kinase effector. Time-resolved X-ray solution scattering data were collected at beamline ID09, as well as at the Advanced Photon Source and the Swiss Light Source.
 |
|
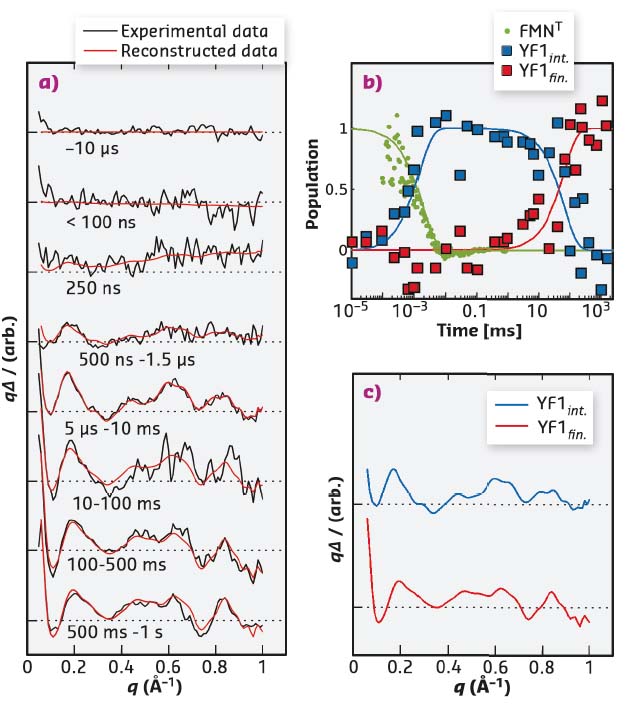
Fig. 56: Time-resolved X-ray solution scattering data (a) can be described by two intermediates following sequential kinetics (b). The time-dependent populations of the intermediates are indicated by squares, and the formation of the photoproduct state as measured by the absorbance at 715 nm is shown in green (b). Time-independent basis spectra are shown in (c). |
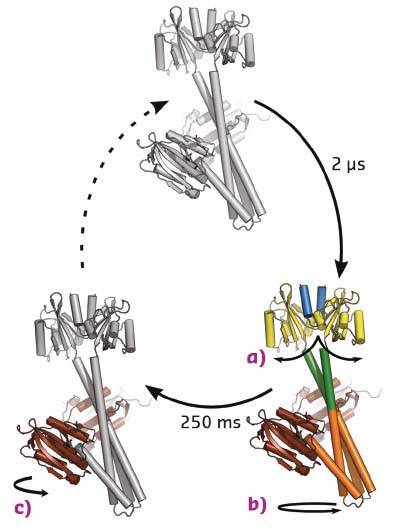
Time-resolved solution scattering reports on the global conformational changes following the absorption of light. Such data were collected by exposing the YF1 protein in solution to a blue laser flash and probing the structural change by X-ray scattering at defined time intervals thereafter. The time-evolution of the difference scattering signal could be described by two sequentially populated states. The difference scattering signals arose with a time constant of 2 µs, essentially simultaneous to the formation of the chromophore photoproduct (Figure 56). Previous studies had shown that upon photoactivation the LOV photosensor dimer splays apart [1,2]. Molecular dynamics simulations were employed to analyse the conformational rearrangements. This structural refinement for full-length YF1 revealed that the separation of the photosensor feeds into the coiled-coil linker as an increase in left-handed supercoiling. A secondary event was observed in the difference scattering data at 250 ms after the laser flash. This event was determined to be an internal reorientation within the histidine kinase effector (Figure 57).
 |
|
Fig. 57: Structural photocycle of YF1. Blue light causes the formation of a flavin-cysteinyl adduct in the photosensory domain. This prompts the splitting of the LOV photosensor dimer (a), which feeds into the coiled-coil linker region as a left-handed supercoiling 2 µs after photon absorption (b). After 250 ms, the effector rearranges internally as the catalytic/ATP-binding domain changes its positioning relative to the central helices (c). |
In summary, the study has revealed global conformational changes in the form of a rotational movement immediately following the formation of the chromophore photoproduct and a secondary rearrangement within the effector domain. The refined changes account for much of the functional data [3] and are in agreement with the emerging views on histidine kinase regulation.
Principal publication and authors
Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase, O. Berntsson (a), R.P. Diensthuber (b), M.R. Panman (a), A. Björling (a), E. Gustavsson (a), M. Hoernke (a,c), A.J. Hughes (a), L. Henry (a), S. Niebling (a), H. Takala (a,d,e), J.A. Ihalainen (d), G. Newby (f), S. Kerruth (g), J. Heberle (g), M. Liebi (h), A. Menzel (h), R. Henning (i), I. Kosheleva (i), A. Möglich (b,j) and S. Westenhoff (a), Nat. Commun. 8, 284 (2017); doi: 10.1038/s41467-017-00300-5.
(a) University of Gothenburg (Sweden)
(b) Humboldt-Universität zu Berlin (Germany)
(c) Albert-Ludwigs-Universität Freiburg (Germany)
(d) University of Jyväskylä (Finland)
(e) University of Helsinki (Finland)
(f) ESRF
(g) Freie Universität Berlin (Germany)
(h) Paul Scherrer Institut, Villigen (Switzerland)
(i) The University of Chicago (USA)
(j) Universität Bayreuth (Germany)
References
[1] C. Engelhard et al., Sci. Reports 7, 1385 (2017).
[2] O. Berntsson et al., Structure 25, 933-938 (2017).
[3] R. Ohlendorf et al., ACS Synth Biol. 5, 1117-1126 (2016).



