- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Complex systems and biomedical sciences
- In vivo permeability increase of brain tumour vessels induced by synchrotron microbeam radiation therapy
In vivo permeability increase of brain tumour vessels induced by synchrotron microbeam radiation therapy
Synchrotron microbeam radiotherapy (MRT), a spatially fractionated preclinical radiotherapy, is more efficient than broad beam irradiation (BB) at opening the blood-brain barrier of all regions of intracranial rodent glioblastomas and it might be used to obtain a better efficacy of adjuvant chemotherapy than with homogeneous radiation fields used in conventional radiotherapy.
The efficiency of blood-borne chemotherapies is hampered by a limited exposure of all the cells in the tumour mass to a sufficient drug concentration due to the anatomic/physiologic characteristics of solid tumours [1]. Exposure to conventional radiotherapy is known to enhance the permeability of brain tumour vessels. Microbeam radiation therapy (MRT), developed on ID17, might enhance vessel permeability specifically in tumours before drug administration [2,3]. This study compares the blood-tumour barrier permeability changes induced by MRT (relying on spatial fractionation of the incident X-ray beam into parallel micron-wide beams), with a spatially non-fractionated radiotherapy.
Male rats bearing intracranial highly malignant F98 gliomas were randomised into three groups: untreated, or exposed to synchrotron microbeams (width 50 μm, 200 μm on-centre spacing, peak and valley dose: 241 and 10.5 Gy respectively) or to broad beam irradiation (BB) delivered at comparable doses (i.e. equivalent to MRT valley dose); both applied by two arrays, intersecting orthogonally in the tumour region. Vessel permeability was monitored in vivo by multiparametric magnetic resonance imaging (MRI) as previously described [4] one day before and one, two, seven and 14 days after treatment start. To find out whether physiological parameters influence vascular permeability, the vessel integrity has been evaluated with different cerebral blood flow, blood volume, oedema and tissue oxygenation values.
 |
|
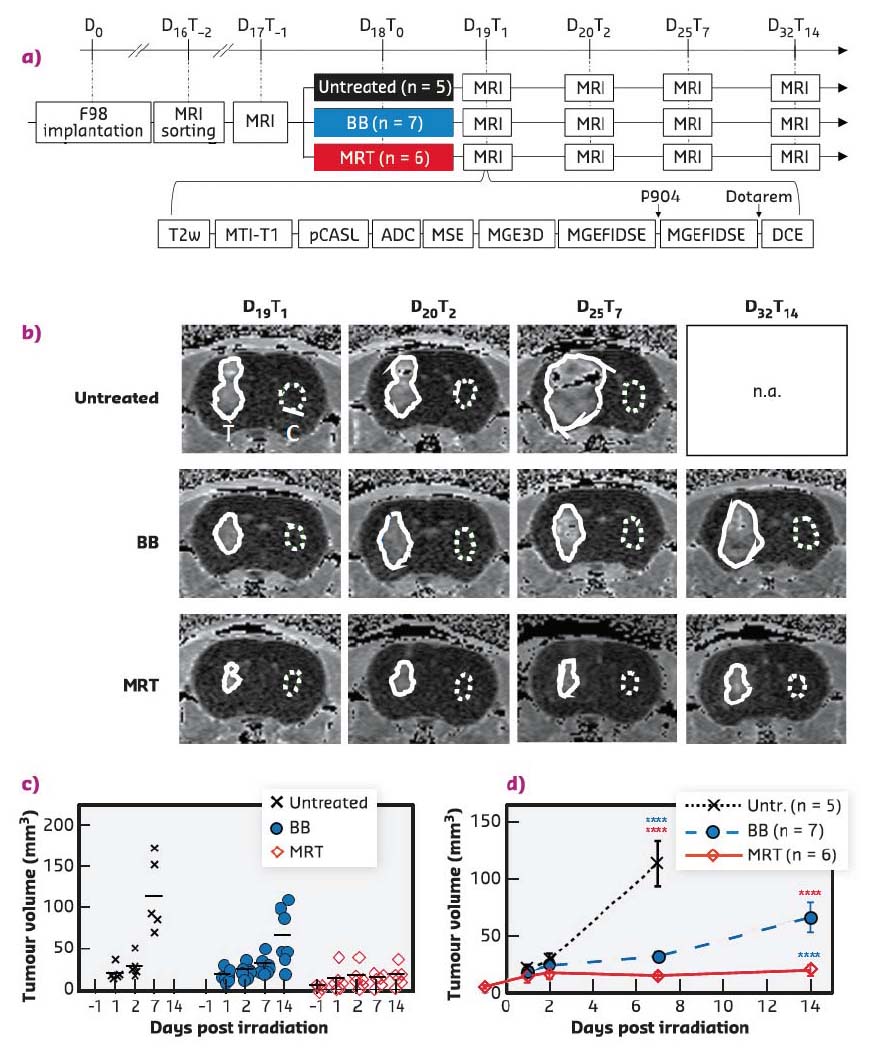
Fig. 66: a) Experimental timeline and diagram representing MRI sequences acquired for each animal at each time point. BB = Broad synchrotron X-ray beam; MRT = Microbeam Radiation Therapy. b) Tumour volume evolution after treatment. MRI follow-up, DCE-enhancement map of an untreated, BB- or MRT-treated F98-bearing rat at different delays after implantation. Solid and dotted lines delineate the tumour and the contralateral ROI, respectively. c) Individual values (plots) and means (lines) and d) means ± SEM tumour volumes measured on DCE-enhancement map at different delays after tumour irradiation for untreated (black), BB- (blue) or MRT-treated animals (red). ****: p < 0.0001. |
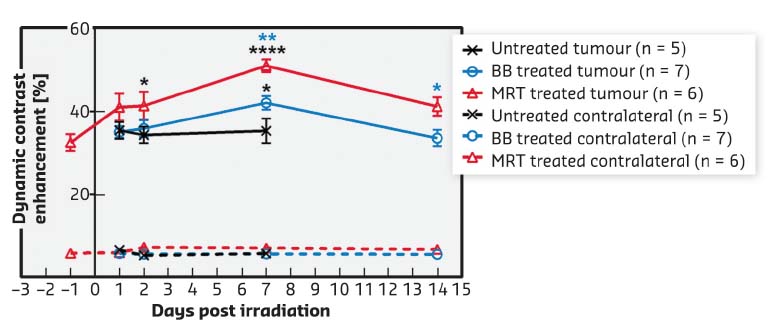
Both radiotherapy modes significantly slowed tumour growth but MRT reduced tumour growth more than BB irradiation did, i.e. tumours were 3.4 times smaller at D32T14 (19.5 ± 11.7 mm3) after MRT than after BB (65.9 ± 34.2 mm3, p < 0.0001) (Figure 66). MRT leads to a significantly higher, earlier and more continuous increase in tumour blood vessel permeability than homogeneous BB irradiation, without affecting healthy tissue (Figure 67). In addition, the study of permeability changes in different areas of the tumour characterised by oedema, blood volume, cerebral blood flow or tissue oxygenation revealed that MRT induces an increase in vascular permeability in all tumour areas, including those tumour areas not impacted by homogeneous irradiation. Finally, it was shown that MRT is more efficient at disrupting brain tumour vessels in the most actively proliferating area of the tumour considered as the relevant target area for adjuvant drug delivery.
 |
|
Fig. 67: Vessel permeability. Dynamic contrast enhancement (DCE) after Gd-DOTA injection measured by MRI in the F98 tumour (solid lines) and normal brain tissue in the contralateral hemisphere (dashed lines) at different times after BB- (blue) or MRT- (red) or no treatment (black). Mean ± SEM. *: p < 0.05; **: p < 0.01; ****: p < 0.0001. |
High-dose microbeams could be used to facilitate the delivery of intravenously injected drugs to tumour tissue. Therefore, adjuvant chemotherapy might be more effective when coupled with MRT than with homogeneous radiation fields used in conventional radiotherapy.
Principal publication and authors
Permeability of Brain Tumor Vessels Induced by Uniform or Spatially Microfractionated Synchrotron Radiation Therapies, A. Bouchet (a), M. Potez (b), N. Coquery (c), C. Rome (c), B. Lemasson (c), E. Bräuer-Krisch (d), C. Rémy (c), J. Laissue (e), E.L. Barbier (c), V. Djonov (a) and R. Serduc (b), Int J Radiat Oncol Biol Phys. 98, 1174-1182 (2017); doi: 10.1016/j.ijrobp.2017.03.025.
(a) Group Topographic and Clinical Anatomy, Institute of Anatomy, University of Bern (Switzerland)
(b) Rayonnement synchrotron et Recherche médicale, Université Grenoble Alpes (France)
(c) Team Functional NeuroImaging and Brain Perfusion, INSERM U1216, La Tronche, Institut des Neurosciences, Université Grenoble Alpes, La Tronche (France)
(d) ESRF
(e) University of Bern (Switzerland)
References
[1] H. Holback and Y. Yeo, Pharm Res. 28, 1819-1830 (2011).
[2] A. Bouchet et al., Int J Rad Oncol Biol Phys. 78, 1503-1512 (2010).
[3] A. Bouchet et al., Phys Med. 31, 634-641 (2015).
[4] B. Lemasson et al., NMR Biomed. 28, 1163-1173 (2015).



