- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Structure of materials
- Resorcinol crystal twisting and polymorphism
Resorcinol crystal twisting and polymorphism
Resorcinol, a commodity chemical intermediate, was the first substance whose crystals showed different arrangements of molecules (polymorphs) by X-ray diffraction. Strange helicoidal resorcinol crystals form under conditions that support a third, ambient-pressure polymorph. The solution of the structure of this phase required powder diffraction data combined with computational crystal structure prediction algorithms.
Resorcinol crystals grow from the melt in the presence of tartaric acid additives as two distinct kinds of optically banded spherulites. The optical banding shown in Figure 59 is evidence of helicoidal twisting of the composite crystallites, a common, non-classical morphology [1]. This very common morphology for molecular crystals was first observed in 1906 for resorcinol [2], but was forgotten until much later [3].
As two forms of resorcinol had been established by X-ray scattering in the 1930s, it was natural to presume that these forms had been induced to twist by the chiral additive. In fact, one was identified by powder X-ray diffraction as the long-known β form of resorcinol [4]. The other phase, not the expected α [5] form, was unknown. We called it ε. It was incumbent upon us to establish that we had indeed found a new ambient phase of resorcinol by solving its structure.
 |
|
Fig. 59: A selection of polarised light optical micrographs of the new ε form of resorcinol. a) Irregular banded spherulite formed at 80°C. b) Banded spherulite formed at 75°C, showing distinct twisting periods. c) Banded spherulite formed at 51°C. Irregular pattern with small areas of banded spherulites formed at 35°C (d). |
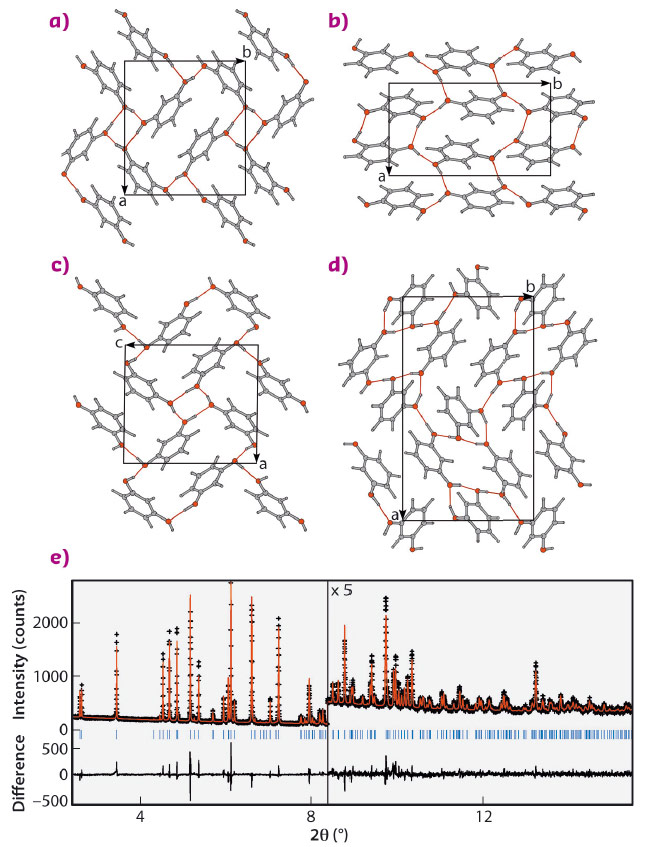
We turned to computation in order to arrive at the crystal structure whose simulated diffraction pattern matched data collected at beamline ID22. Several crystal structure prediction attempts have been made, one of which applied a resorcinol-specific force field for energy minimisation [6]. Ultimately, the structure was matched by the USPEX evolutionary algorithm [7]. In addition to β (Figure 60b) and ε (Figure 60c), there was one other structure that was found with 5 kJ/mol of the a (Figure 60a) ground state. This was a monoclinic structure as shown in Figure 60d, a target of future experimental efforts.
 |
|
Fig. 60: Resorcinol crystal structures a) α, b) β, c) ε, and d) another low energy phase in the space group P21, not observed. The views in each case are such that the molecules in any one structure project equal areas onto the plane of the page. e) Rietveld refinement of high-resolution synchrotron powder diffraction for the ε resorcinol sample containing 5 wt.% (2S,3S)-(-)-tartaric acid crosses) and calculated (red line). Rwp = 7.74%, χ2 = 0.838. Data were collected at beamline ID22 at a wavelength of 0.41064 Å and at a temperature of 200 K. The lower trace shows the difference curve. |
Resorcinol has been so thoroughly studied in the past 80 years that some researchers were of the opinion that no additional forms could be found under ambient conditions [8], a prediction that proved to be premature.
Many polymorphs of common substances can be found under highly non-equilibrium conditions that are well suited to the unusual twisted morphologies that characterise resorcinol. New regions of phase space can be reached when the driving force for crystallisation is particularly high. Nevertheless, phases prepared in this way are typically polycrystalline, and require high-resolution powder X-ray diffraction filtered through the prism of computation. Despite claims that crystal structure prediction on the basis of molecular structure alone is a solved problem, the challenges raised by the simple case of resorcinol demonstrates that such is not the case.
Principal publication and authors
Resorcinol crystallization from the melt: a new ambient phase and new “riddles”, Q. Zhu (a), A.G. Shtukenberg (b), D.J. Carter (c), T.-Q. Yu (b), J. Yang (b), M. Chen (b), P. Raiteri (c), A.R. Oganov (a), B. Pokroy (d), I. Polishchuk (d), P.J. Bygrave (e), G.M. Day (e), A.L. Rohl (c), M.E. Tuckerman (b,f) and B. Kahr (b), J. Am. Chem. Soc. 138, 4881–4889 (2016); doi: 10.1021/jacs.6b01120.
(a) Stony Brook University (USA)
(b) New York University (USA)
(c) Curtin University (Australia)
(d)Technion Israel Institute of Technology (Israel)
(e) University of Southampton (UK)
(f) New York University Shanghai (China)
References
[1] A. Shtukenberg et al., Angew. Chem., Int. Ed. 53, 672 (2014).
[2] F. Wallerant, C. R. Acad. Sci. (Paris) 143, 555 (1906).
[3] B. Kahr et al. Rohl, Cryst. Growth Des. 11, 2070 (2011).
[4] J.M. Robertson and A.R. Ubbelohde, Proc. R. Soc. London, Ser. A: Math. Phys. Sci. 167, 136 (1938).
[5] J.M. Robertson and A.R. Ubbelohde, Proc. R. Soc. London, Ser. A: Math. Phys. Sci. 167, 122 (1938).
[6] J. Chatchawalsaisin et al. CrystEngComm 10, 437 (2008).
[7] A.R. Oganov et al., Acc. Chem. Res. 44, 227 (2011).
[8] K. Druzbicki et al., J. Phys. Chem. B 119, 1681 (2015).



