- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Electronic structure, magnetism and dynamics
- Operando X-ray methods illuminate the way towards catalyst active site design for methane conversion
Operando X-ray methods illuminate the way towards catalyst active site design for methane conversion
The upgrading of methane could prove a valuable alternative to oil as a source of platform chemicals. A combination of X-ray methods under real reaction conditions has provided detailed new insight into the evolution of Mo species during the catalytic conversion of methane and its influence on the reaction products.
With oil resources becoming progressively scarce, the chemical and petrochemical industry will undergo a major transformation in the coming decades. Alternative raw materials (e.g. coal, natural gas, biomass or CO2) will be required, enforcing the development of step-change technologies for the large-scale production of platform chemicals. Within this context, the increasing availability of cheap natural gas has attracted growing interest towards direct routes for methane upgrading [1]. One of these routes, methane dehydroaromatisation (MDA), is particularly promising for the direct conversion of methane into aromatics and hydrogen using metal-exchanged zeolites such as Mo/H-ZSM-5, since it contains acid sites as well as Mo species possessing dehydrogenation and C-C coupling functionalities [1-2]. It is generally accepted that methane is activated on the exchanged Mo species, forming ethylene. Subsequently, ethylene reacts on the Brønsted acid sites and converts into aromatics, also leading to coke formation by consecutive reaction of aromatics with light olefins. While active species are proposed to originate from either (MoO2)2+ monomers or (Mo2O5)2+ dimers, there is also a debate on whether the active sites are oxidic, carbidic (MoCx) or oxycarbidic (MoCxOy) in nature. In addition, there is no clear understanding of the mode of catalyst deactivation, considered the main limitation for the commercialisation of the process [1-2].
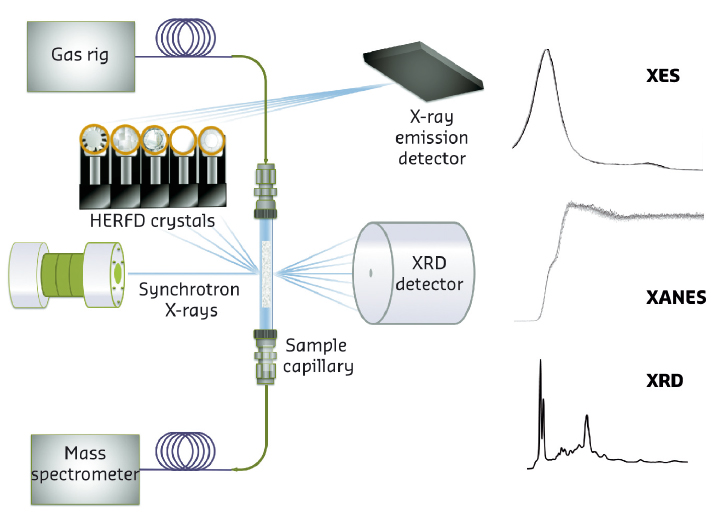
A rational design and tailoring of an improved catalyst can only be realised by understanding how it actually operates and deactivates under reaction conditions. Using a novel combination of techniques, coupling for the first time high energy resolution fluorescence detection X-ray absorption near-edge structure (HERFD-XANES) spectroscopy, X-ray difracction (XRD), and X-ray emission (XES) under real operando conditions at beamline ID26 (see Figure 21), we have been able to correlate the evolution of the Mo species with the distinct reaction products formed during the methane dehydroaromatisation (MDA) reaction. Of particular importance has been the application of the scarcely-used X-ray emission spectroscopy (XES), which is able to distinguish between ligands surrounding metal ions when they possess a similar Z number, i.e. C vs O. In this work, we used the Mo Kβ valence-to-core emission lines in conjunction with HERFD-XANES spectroscopy in order to be able to unambiguously determine the existence of both MoCx and MoCxOy during the course of reaction.
 |
|
Fig. 21: Schematic representation of the setup used at ID26. |
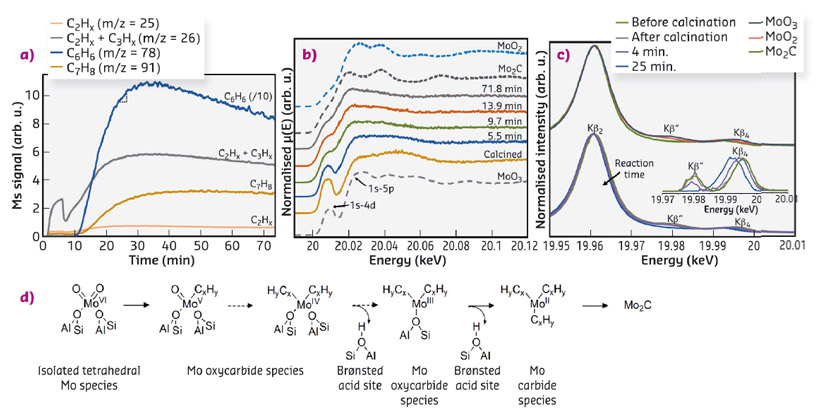
Based on the operando HERFD-XANES/XES results (Figure 22a-c), a complete pathway for the evolution of Mo was obtained, and correlated with the distinct reaction products detected by online mass spectrometry. As illustrated in Figure 22d, isolated Mo-oxo centres present after calcination are seen to be converted by methane into metastable MoCxOy species, which are primarily responsible for the formation of light hydrocarbons. These are still attached to the zeolite framework and present varying stoichiometry depending on the extent of the carburisation. Longer reaction times eventually lead to the transition to a Mo carbide phase, involving the formation of MoC3 sites not connected to the framework, whose presence coincides with benzene formation. As such, these MoC3 species are not stable and easily agglomerate. Ultimately, sintering of MoC3 leads to the migration of the clusters to the outer zeolite surface and the formation of larger Mo2C-like nanoparticles.
 |
|
Fig. 22: a) Mass spectrometry data. b) Operando Mo K-edge HERFD-XANES spectra. c) Kβ valence-to-core emission lines. d) Evolution of Mo species. |
Further insight into the catalyst deactivation mode was obtained from the operando XRD data and complementary fluorescence lifetime microscopy measurements, which evidenced the formation of large hydrocarbons on the external zeolite surface, allowing us to conclude that both sintering of MoC3 and the accumulation of carbon deposits on the outer shell of the zeolite crystals are primarily responsible for the decrease in catalytic performance.
Overall, these results demonstrate the importance of controlling, or else maintaining, Mo active species to achieve the desired product formation, which has important implications for realising the impact of methane as a source for platform chemicals. Although transient, the appeal of stabilising MoCxOy species is that they are highly selective to light hydrocarbons. Whilst there are issues with stabilising MoCxOy in the presence of the reaction mixture, this could be achieved in the presence of co-fed H2O and/or O2. MoC3 species in turn are the species to target for aromatics but again either co-feeding of oxidants to mitigate carbon deposition or else enhancing the interaction with the zeolite is needed for improved stability.
Principal publication and authors
Molybdenum speciation and its impact on catalytic activity during methane dehydroaromatization in zeolite ZSM-5 as revealed by operando X-ray methods, I. Lezcano-González (a), R. Oord (b), M. Rovezzi (c), P. Glatzel (c), S.W. Botchway (d), B.M. Weckhuysen (b) and A.M. Beale (a), Angew. Chem. Int. Ed. 55, 5215 (2016); doi: 10.1002/anie.201601357.
(a) UK Catalysis Hub, Research Complex at Harwell & University College London (UK)
(b) Utrecht University (The Netherlands)
(c) ESRF
(d) Central Laser Facility (UK)
References
[1] J.J. Spivey and G. Hutchings, Chem. Soc. Rev. 43, 792 (2014).
[2] Z.R. Ismagilov et al., Energy Environ. Sci. 1, 526 (2008).



