- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Complex systems and biomedical sciences
- Watching quantum dots grow in real time
Watching quantum dots grow in real time
Time-resolved SAXS/WAXS at beamline ID02 was used to unravel the steps of nanoparticle nucleation and growth from molecular precursors in an in situ experiment.
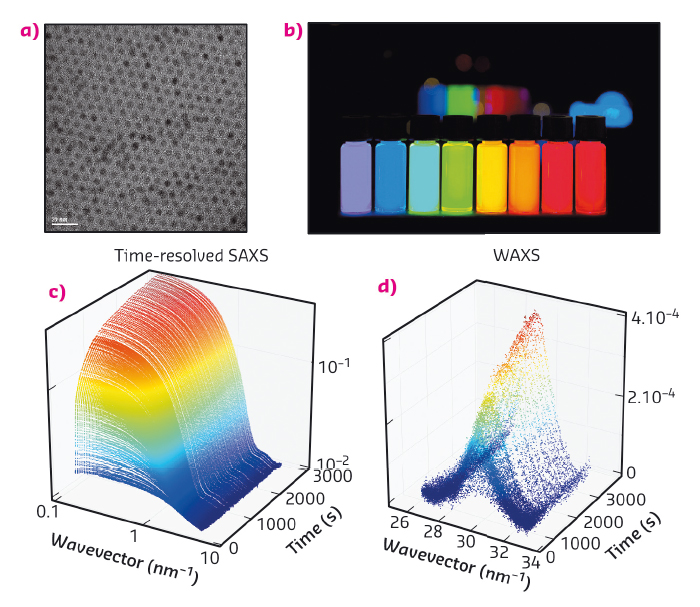
Discovered in the early eighties, quantum dots are nanoparticles composed of a crystalline core surrounded by a layer of ligands (Figure 83a) [1-4]. Their core is made of a semi-conducting material whose band-gap varies with the size of the particle due to a confinement effect. In the case of CdSe, these particles are fluorescent in the visible range and emit a bright, intense light when illuminated by UV radiation (Figure 83b). The wavelength at which the quantum dots emit only depends on their size: from 1 nm for a blue emitting particle to 5 nm for red (Figure 83b). These outstanding optical properties can be exploited in diverse applications such as light-emitting devices, biological imaging or displays. Interestingly, TVs containing quantum dots exhibit superior colour quality to existing technologies and will soon hit the market.
 |
|
Fig. 83: (a) Electron microscopy image of CdSe quantum dots. (b) Vials containing a dispersions of quantum dots under UV illumination. From left to right, the size of the quantum dots increases from 1 to 5 nm. (c) and (d) Sequence of time-resolved SAXS and WAXS diagrams obtained in situ during the formation of quantum dots. |
Making these nanoparticles requires mixing and heating inorganic precursors with surfactants in an organic solvent. Though a lot of these recipes are now available in the literature, optimising existing synthesis or discovering new nanoparticles with desired shape or composition is a lengthy process demanding tedious variation of the experimental parameters. Our mechanistic understanding of nanocrystal formation is still in its infancy and only recently efforts have been put towards this. Similar to other fields of synthetic chemistry, deeper mechanistic insight will accelerate the discovery of new products and enable larger-scale syntheses. From a fundamental standpoint, various models have been proposed for the nucleation and growth of nanocrystals but experimental evidence lags behind.
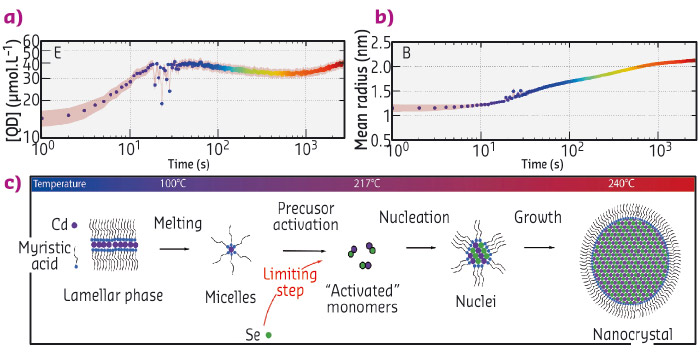
Acquiring experimental data during the formation of the particles is a difficult experimental challenge since it requires the structure of the solution to be probed on small time (below the second) and length scales (nanometre). Here, we used time-resolved small and wide angle X-ray scattering at beamline ID02 to probe the formation of quantum dots in real time. We chose a synthesis of quantum dots where the precursors are mixed at ambient temperatures and heated to 240°C [5]. We mounted a precursor filled capillary on a heating stage and heated it while acquiring SAXS and WAXS patterns (Figure 83c and d) during the course of the reaction. Before the onset of nanoparticle formation, we found that the inorganic precursors self-organised into a lamellar phase which melts at 100°C. We detected the appearance of the first nanoparticles at 173°C. The SAXS signal was fitted to a model of polydisperse spheres using a Monte-Carlo algorithm [6]. From these fits, we extracted the mean radius and polydispersity as well as the nanocrystal concentration (Figure 84a and b). This analysis reveals that the formation mechanism can be decomposed into two stages: a nucleation phase during which new particles appear that is followed by a growth phase during which the nanocrystal concentration is almost constant while the mean radius increases. We compared our experimental data to classical theoretical models (diffusion or reaction limited growth) and proved that growth was in fact limited by the supply of soluble selenium (Figure 84c).
 |
|
Fig. 84: (a) and (b) Concentration of nanoparticles and mean radius obtained from the Monte-Carlo fitting of the SAXS patterns. (c) Scheme describing the formation mechanism of the quantum dots. |
To address the crystalline structure of the nanocrystals during their formation, WAXS patterns were also acquired. The (220) peak of the zinc blende structure was fitted to a gaussian line shape. Surprisingly, the position of the peak shifted towards smaller q values with time indicating an expansion of the crystal lattice as the nanoparticle grow. The physical basis of the phenomenon is not clear yet and its comprehension requires further studies.
These results demonstrated the feasibility of studying quantum dot formation in situ at high temperatures. Future work will address the effect of the variation of the different experimental parameters and anisotropic growth of nanoparticles such as 2D nanoplatelets.
Principal publication and authors
Real-time in situ probing of high-temperature quantum dots solution synthesis, B. Abécassis (a), C. Bouet (b), C. Garnero (a), D. Constantin (a), N. Lequeux (b), S. Ithurria (b), B. Dubertret (b), B.R. Pauw (c) and D. Pontoni (d), Nano Lett. 15, 2620–2626 (2015); doi: 10.1021/acs.nanolett.5b00199.
(a) Laboratoire de Physique des Solides, Univ. Paris-Sud, CNRS, UMR 8502, Orsay (France)
(b) LPEM, ESPCI-ParisTech, PSL Research University, CNRS UMR 8213, Sorbonne Universités, UPMC Paris 06, Paris (France)
(c) National Institute for Materials Science (NIMS), Tsukuba (Japan)
(d) ESRF
References
[1] L.E. Brus, J. Chem. Phys. 80, 4403 (1984).
[2] A.I. Ekimov et al., Solid State Commun. 56, 921 (1985).
[3] A.P. Alivisatos, Science 271, 933 (1996).
[4] Y. Yin, A.P. Alivisatos, Nature 437, 664 (2005).
[5] Y. Yang et al., Angew. Chem. Int. Ed. 44, 6712 (2005).
[6] B.R. Pauw et al., J. Appl. Crystallogr. 46, 365 (2013).



