- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structure of materials
- Multinary thermoelectrics – a challenge for crystal structure determination
Multinary thermoelectrics – a challenge for crystal structure determination
The conversion of thermal into electrical energy is an important goal for better energy efficiency by means of waste-heat recovery. Modern thermoelectric materials render this conversion possible and gained worldwide interest because of their high ‘thermoelectric figures of merit’ ZT, which depend on the thermal (κ) and electrical (σ) conductivity, the Seebeck coefficient (S) and the temperature (T): ZT = (S2σ/κ)T. State-of-the-art bulk materials often contain a mixture of elements with very low scattering contrast like Pb and Bi or Ag, Sn, Sb and Te [1], which often share atomic sites in the corresponding crystal structures. These elements can reliably be distinguished by the effect of anomalous dispersion at absorption edges, where the dispersion correction term Δf’ is large and strongly modifies the atomic form factor f(λ,θ) = f 0(sinθ/λ) + Δf’(λ) + iΔf’’(λ). These correction terms can be determined directly at the beamline: Δf’’(λ) is calculated from X-ray fluorescence and Δf’(λ) can be derived from it. The scattering contrast between neighbouring elements in the periodic table can be enhanced by up to 8 electrons [2].
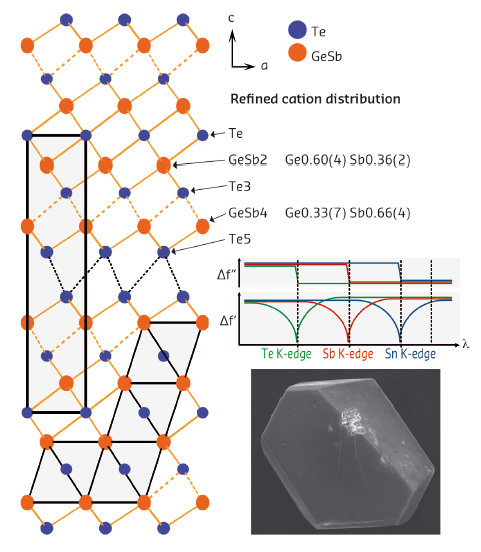
Beamline ID11 is ideally suited to high-resolution single crystal diffraction because of its stable beam and small band pass. A fluorescence detector for the determination of dispersion correction terms as well as a furnace for high-temperature measurements are integral parts of the setup used to refine the element distribution and structural changes in Ge2Sb2Te5 as a function of temperature [3]. This material is well known as a fast phase-change material for data storage. Its stable phase consists of tetradymite-like distorted rocksalt-type slabs which are terminated by Te-atom layers and separated by van der Waals gaps (Figure 129). The element distribution changes very little upon heating, but instead the GeTe-like layers detach reversibly from the slab. In addition, this class of materials turned out to exhibit very promising thermoelectric properties which led to many substitution experiments to further enhance the efficiency of energy conversion.
 |
|
Fig. 129: Room-temperature structure of Ge2Sb2Te5 (left) with refined element distribution (top right); the principal trend of the dispersion correction terms is sketched (middle right); bottom right: SEM image of the crystal investigated. |
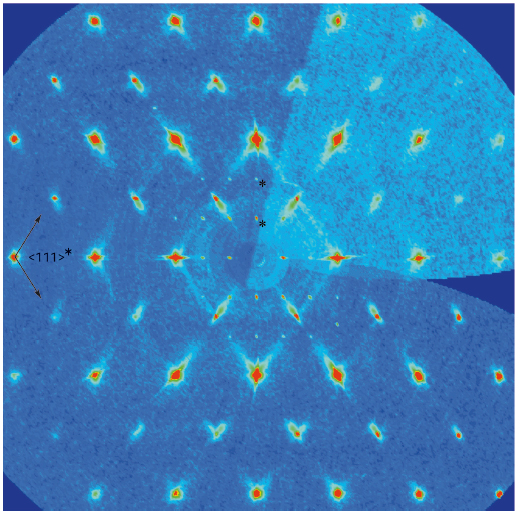
Ge~5Sb2(Te0.13Se0.87)~8, for example, crystallises in a highly disordered rocksalt-type structure. TEM investigations revealed that the vacancies tend to order in parallel non-equidistant defect planes (111). This involves fourfold twinning and stacking disorder, which is also corroborated by diffuse streaks in the diffraction patterns obtained at beamline ID11 (Figure 130). Semi-quantitative information on the real structure can be drawn by comparing the diffraction patterns of crystals with different compositions (GeTe)nSb2(SexTe1-x)3 and simulated patterns. The most promising thermoelectric properties (ZT = 1.2 at 425°C) were found for n = 7 and x = 0.2. Furthermore, the refinement of the atom distribution in long-range ordered layered compounds like Ge0.6Sn0.4Sb2Te4 [4] yields clear statements concerning the most probable local structures in disordered compounds. Occupancy factors on sites shared by Sn, Sb and Te, impossible to distinguish using laboratory X-ray sources, could be determined in a joint refinement on five datasets (three at the K-edges of Sn, Sb and Te, one further off-edge synchrotron and one laboratory dataset).
 |
|
Fig. 130: Reciprocal lattice section (hhl) from a crystal of Ge~5Sb2(Te0.13Se0.87)~8; note the diffuse scattering running along <111>* (λ/3 reflections are marked with asterisks). |
In conclusion, the use of anomalous dispersion is a unique way to reliably determine structures of promising thermoelectric materials that contain several elements with a low scattering contrast. In situ investigations and the refinement of site occupancies of mixed sites are easily possible.
Principal publication and authors
T. Rosenthal (a), P. Urban (b), K. Nimmrich (a), L. Schenk (a), J. de Boor (c), C. Stiewe (c) and O. Oeckler (b), Chem. Mater. 26, 2567-2578 (2014).
(a) Department of Chemistry, LMU Munich (Germany)
(b) Faculty of Chemistry and Mineralogy, Leipzig University (Germany)
(c) German Aerospace Center, Cologne (Germany)
References
[1] L.-D. Zhao, V.P. Dravid and M.G. Kanatzidis, Energy Environ. Sci. 7, 251-268 (2014).
[2] S. Welzmiller, P. Urban, F. Fahrnbauer, L. Erra and O. Oeckler, J. Appl. Crystallogr. 46, 769-778 (2013).
[3] P. Urban, M.N. Schneider, L. Erra, S. Welzmiller, F. Fahrnbauer and O. Oeckler, CrystEngComm 15, 4823-4829 (2013).
[4] S. Welzmiller, T. Rosenthal, P. Ganter, L. Neudert, F. Fahrnbauer, P. Urban, C. Stiewe, J. de Boor and O. Oeckler, Dalton Trans. 43, 10529-10540 (2014).



