- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Enabling technologies
- The ESRF Helium network
The ESRF Helium network
Helium is the second-lightest element in the Universe (seven times lighter than air), it has the lowest boiling-point, the lowest liquefaction temperature (-269°C) and, unlike any other element, helium will remain liquid down to absolute zero at ambient pressures. Its thermal conductivity and transmission of speed of sound are superior to that of all other gasses (save hydrogen). It is also the least water-soluble of all gasses. Colourless, odourless and non-toxic, helium is particularly chemically inert and ranks as the second least reactive noble gas, after neon. The remarkable properties of this gas have led to its widespread use in both liquid and gas forms.
In industry, helium is used to inflate airships and weather balloons. It provides a protective atmosphere in the manufacture of integrated circuits, screens, and in arc welding procedures. Helium can be used as a leak detector in vacuum equipment or in high pressure tanks, or to determine water tightness in food packaging. In science, helium is an integral element in cryogenic procedures for cooling or for experiments that require extremely low temperatures (supra conductors). It is also used in optics for its refraction index and in the aeronautics industry, laser systems and leisure activities (inflating balloons).
Helium is an irreplaceable but non renewable gas. It is the most abundant element in the universe, second only to hydrogen, but it is much rarer on earth. There is no synthesising method capable of producing helium except for nuclear fusion. Helium is a sub-product of natural gas that contains it from some parts per million to several percent. Helium-producing countries are USA (78%), Algeria, Qatar, Russia and Poland.
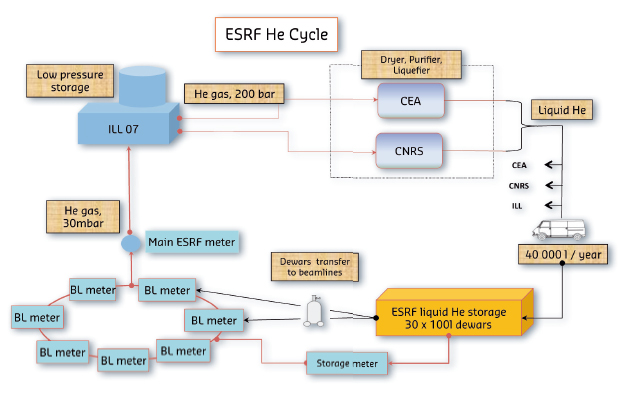
In 1996, the US passed a law which diminished the return on investment of recycling helium; the law stated that the US National Helium Reserve, which is kept in a disused underground gas field in Texas – by far the biggest store of helium in the world – must all be sold off by 2015, irrespective of the market price. The ESRF applies a standard practise for distribution and recovery of helium. ESRF benefits from compressed gas storage facilities at the ILL and the liquefaction services available at the CNRS and the CEA. Liquid helium is supplied by the CEA or CNRS in 100 dewars. Users connect the dewars to the equipment to be cooled (cryostats) then connect the gas outlet to the helium recovery network (50 mm stainless steel tube), running for 1.5 km around the storage ring and to the ILL.
An individual meter on each beamline makes it possible to control the volume of gas transferred over the network. This low pressure gas (about 30 mbar) is fed back into the ILL system where it is stored in a buffer tank (volume 20 000 l) then compressed into bottles at about 200 bars. The compressed gas is then transported to one of the liquefaction centres in either the CEA or CNRS, where it goes through a drying and purification process at low temperature (77 K, -196°C) to separate oxygen, nitrogen and water vapour. It is then re-liquefied (passage from gas phase to liquid phase) to fill the dewars which come back again to supply the ESRF experiments (Figure 155).
 |
|
Fig. 155: The helium recovery process at the ESRF. |
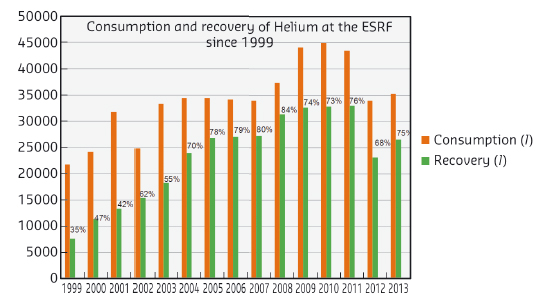
The ESRF’s consumption of liquid helium is about 40 000 per year with a recovery rate of over 70% (see Figure 156). An analysis of the recovery rate shows that it can vary between 50 and 80% depending on the month and the year. The major factors causing helium loss are evaporation (about 10%) and accidental loss (leaks on experimental pipes, poor connections). Nonetheless, in case of an important contamination of the recovered gas (e.g. introduction of air), the global volume of recovered helium is affected because the correct functioning of the liquefier is linked to the purity of the gas (at very low temperatures solidified gas particles can cause damage).
 |
|
Fig. 156: Consumption and recovery of helium at the ESRF since 1999. |
Adhering to simple procedures reduces loss and improves the recovery rate. Dewars pending use are systematically connected to the recovery network; dewars must be returned rapidly after use at a cold temperature, i.e. not completely empty. The tightness of the connections between the dewars and the experiment and the recovery network must be checked. Indications on the recovery meter should be checked against consumption. A significant difference could indicate a leak or contamination by ambient air and a local contamination could shutdown the whole of the ESRF recovery network.
Authors
F. Favier, G. Guernet and T. Marchial.
ESRF



