- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- X-ray imaging
- Hard X-ray nanoprobe investigations of the sub-tissue metal distributions within Daphnia magna
Hard X-ray nanoprobe investigations of the sub-tissue metal distributions within Daphnia magna
The freshwater crustacean Daphnia magna is an important and frequently used model organism to investigate toxicity, uptake, elimination and detoxification processes associated with the exposure to transition metals (e.g. Cu, Zn, Ni) [1-2]. Since biological effects due to metals are initiated by metal bioaccumulation, the fundamental processes underlying bioaccumulation will lead to an improved capacity to evaluate the impact of metals on aquatic organisms [3]. Aquatic biota regulate their internal concentrations of essential metals through active regulation, storage/detoxification or a combination of both. To fully understand these processes, advanced trace level elemental imaging techniques are necessary as conventional bulk elemental analysis methods lack the ability to distinguish the accumulation in critical tissues from whole body accumulation [3]. Since our ultimate aim was to clarify some of the regulation processes by which metals influence the aquatic biota, the metal intoxication needs to be characterised by studying the tissue specific distribution of metals and also by determining their distribution at the sub-tissue level.
We performed exploratory experiments to study the tissue specific distribution of metals on the sub-micrometre scale using the intense nanometre sized hard X-ray beam at the XRF-nanoprobe of beamline ID22NI. Elemental distributions of trace concentration levels could be determined with unprecedented spatial resolution by scanning nano-XRF techniques, which can be translated into the quantitative distribution of metals and metalloids at the sub-microscopic level. With the aim of shifting the ecotoxicological research on D. magna from the microscopic tissue scales towards a (sub) cellular field of view, thin tissue sections of the crustacean D. magna were then analysed. Since nano-XRF has never been performed on Daphnia thin-sections, our measurements are the first to shed light on the sub-tissue metal distributions within these organisms, cultured under controlled exposure conditions. The laboratory clone juveniles (0-48h) of Daphnia magna Straus (clone K6), used in all our experiments, was originally collected from a pond in Kiel (Antwerp, Belgium) and has been successfully cultured under controlled laboratory conditions for more than 10 years. The basis exposure medium consisted of 0.077 mmol/L NaHCO3, 0.078 mmol/L KCl, 0.5 mmol/L MgSO4. The experiment was designed to investigate the accumulation of Zn at a concentration level of 1 mg/L, which caused a 50% mortality of the population after 48 hours of exposure.
 |
|
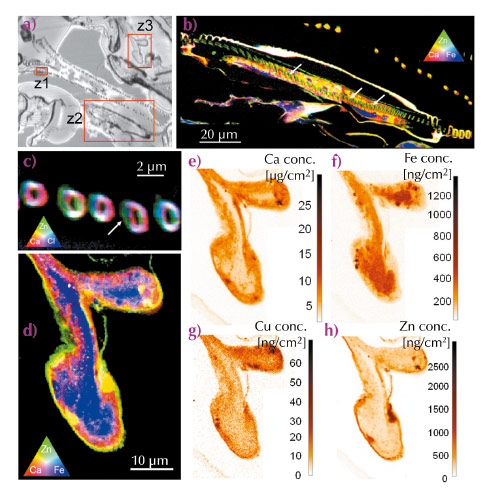
Fig. 24: a) Optical image of an unstained thin section (2 µm) of gill tissue of D. magna; b) elemental distribution maps of Ca, Fe and Zn in the exopodite (region z2); c) Ca, Zn and Cl distributions within the filter-plate (region z1); d) Ca, Zn and Fe distributions within the epipodite (region z3); e-h) areal concentration maps of Ca, Fe, Cu and Zn detected within the epipodite (region z3). |
Figure 24 shows an optical microscope image (a) of an unstained thin section (thickness 2 µm) of the osmoregulatory gill tissue of Daphnia magna, together with several scanning nano-XRF maps associated with the indicated sub-regions (z1, z2, z3), corresponding to the sub-tissue elemental distributions of Ca, Fe, Zn and Cl. Figure 24b and c show colour-coded (RGB) representations of the exopodite and filter plate regions with the conjoint presence of Ca (red), Zn (green) and Cl (blue). Interestingly, a cross section of an epipodite was present within this dorsoventral section, allowing the investigation of the distribution of cations and anions within this osmoregulatory gill tissue. Figure 24d shows an RGB representation of the conjoint presence of Ca, Zn and Fe within the epipodite. The marked presence of Fe confirms the haemolymph flowing through this tissue. This iron rich ‘vesicle’ is enclosed by respectively the Ca containing exoskeleton and a thin Zn rich layer in the micrometre range. These observations corroborate previous ecotoxicological research in which the accumulation of Zn within the exoskeleton was suggested [1]. The elevated levels of Zn could be clearly detected and quantified in the analysed regions, corresponding to concentration levels up-to 2500 ng/cm2. The fast-scanning nano-XRF technique (typical acquisition time/pixel was 100-200 ms, pixel size 100-200 nm) was well-suited to trace level bio-imaging, demonstrating the potential of providing new insights in the cellular detoxification processes within these types of biological model organisms. Our results also illustrate that trace level chemical imaging using scanning X-ray fluorescence analysis is progressing towards the nanoscopic scales, advancing our understanding concerning the role of metals in ecotoxicology.
Principal publication and authors
B. De Samber (a), K.A.C. De Schamphelaere (b), C.R. Janssen (b), B. Vekemans (a), R. De Rycke (c), G. Martinez-Criado (d), R. Tucoulou (d), P. Cloetens (d) and L. Vincze (a), Anal. Bioanal. Chem. 405, 6061-6068 (2013).
(a) Department of Analytical Chemistry, Ghent University (Belgium)
(b) Laboratory of environmental toxicology and Applied Ecology, Department of Applied Ecology and Environmental Biology, Ghent University (Belgium)
(c) Department of Molecular Biomedical Research, Flanders Institute for Biotechnology (VIB), Belgium and Department of Biomedical Molecular Biology, Ghent University (Belgium)
(d) ESRF
References
[1] B. Muyssen and C. Janssen, Arch. Environ. Contam. Toxicol. 43, 492-496 (2002).
[2] K. De Schamphelaere and C. Janssen, Environ. Sci. Technol. 38, 6201-6209 (2004).
[3] B. De Samber, G. Silversmit, K. De Schamphelaere, R. Evens, T. Schoonjans, B. Vekemans, C. Janssen, B. Masschaele, L. Van Hoorebeke, I. Szaloki, F. Vanhaecke, K. Rickers, G. Falkenberg and L. Vincze, J. Anal. At. Spectrom. 25, 544-553 (2010).



