- Home
- News
- General News
- Sea squirt solves...
Sea squirt solves crystal conundrum
12-07-2013
The ESRF has helped crack the crystal structure of vaterite, a rare form of calcium carbonate that has perplexed scientists for almost a century.
Calcium carbonate is one of nature’s most abundant raw materials, much of it formed from the remains of microscopic sea creatures. It comes in three anhydrous crystalline polymorphs. Calcite, which has a trigonal symmetry, is the most stable and abundant form. Aragonite, shown to be orthorhombic by none other than William Bragg a century ago, is metastable and transforms into calcite on geological timescales. The third form, vaterite, named after German mineralogist Heinrich Vater, is the rarest and least stable form of anhydrous calcium carbonate.
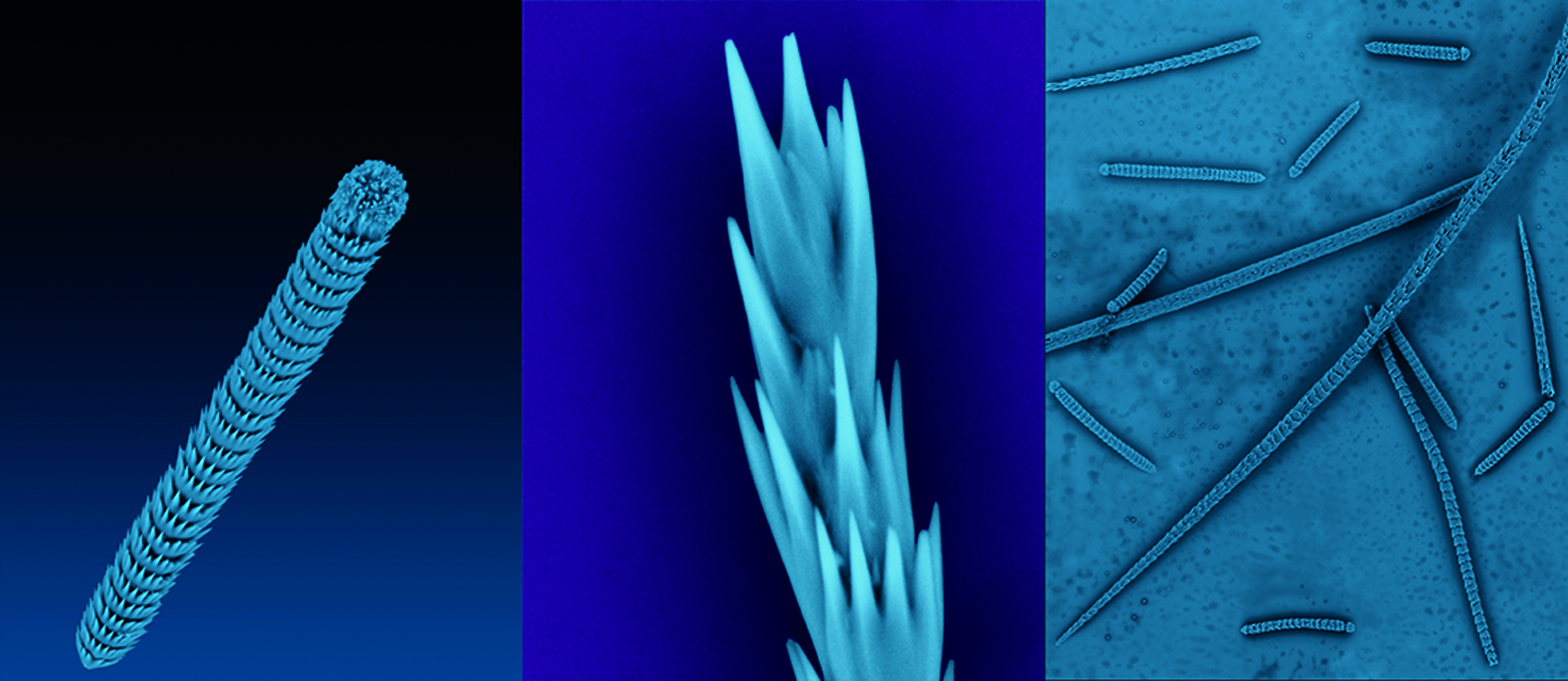
Spicules from the simple sea creature Herdmania momus contain large single crystals of vaterite of higher quality than those in the synthetic vaterite used in previous structure determinations.
Vaterite is an important constituent of cement, where the addition of water transforms it into calcite, and also in the paper industry where it is used as a filler to provide colour or texture. Unlike the other calcium carbonate polymorphs, however, the crystal structure vaterite has eluded researchers for almost a century.
Although vaterite has been found during oilfield drilling, in gallstones and even in a meteorite, geological vaterite is rare and unstable. The synthetic version, a powder, yields only nanosized crystal grains. Biogenic vaterite is produced as a minority component in green turtle eggshells, freshwater pearls and the scar tissue deposited by mollusks to repair their shells. But one creature in particular, a solitary filter-feeding marine invertebrate called Herdmania momus, has a body and tunic spicules made entirely of pure vaterite.
Now, thanks to X-ray powder diffraction at the ESRF and high-resolution transmission electron microscopy, this humble “sea squirt” has given up vaterite’s structure – with unexpected results. The data show that vaterite does not have one crystal form but two different structures that coexist within a pseudo-single crystal (Science 340 454). “We never envisaged this scenario,” explains Boaz Pokroy of the Technion-Israel Institute of Technology, who led the study. “It was a total surprise, but at the same time it made so much sense knowing the years of conflicting results from different groups publishing on the structure of vaterite.”
Structural riddle
Researchers thought they had solved vaterite’s structure as long ago as 1925, when X-ray diffraction revealed hexagonal symmetries that made it distinct from calcite or aragonite. As better tools came along, including Raman spectroscopy, this picture needed to be refined. In the 1950s vaterite was linked to a unit cell with pseudo–hexagonal-orthorhombic symmetry, but in 1963 researchers reported eight weak reflections that were attributed to a superstructure rotated 30° from the main hexagonal one. Since then, the debate about vaterite’s structure has centered on whether its symmetry is hexagonal or orthorhombic, with researchers unable to reconcile diffraction data with optical spectroscopy and also with density functional theory. “In latter years there have been more attempts to solve the structure of vaterite within the concept that it is a single structure,” says Pokroy.
In 2011 Pokroy and co-workers grinded a multitude of Herdmania momus spicules obtained from the Mediterranean and the Red Sea into a fine powder and placed it in the ESRF’s ID31 beamline. None of the previously reported vaterite structures could fit the high-resolution diffraction spectrum, they found. While a hexagonal structure gave the best fit to the data, says Pokroy, it did not explain all the observed diffraction peaks. “ID31 really proved that all the structures were simply wrong – our results change the concept of vaterite.”
To ensure that the data had not come from other structures, such as aragonite or calcite, the team used aberration-corrected high-resolution transmission electron microscopy at the Technion to examine very small volumes of vaterite. This confirmed its dual-crystal structure, but the precise nature of the second structure – which is less abundant than the larger hexagonal form and visible only in certain orientations – remains unknown.
This year, the team intends to remedy that situation using diffuse scattering at the ESRF’s ID13 beamline in collaboration with Christian Riekel. The microfocussing capabilities of this beamline allow a single Herdmania momus spicule to be studied, with higher statistics allowing the team to map the diffraction spots coming from the second crystal. “Although this study is a basic scientific work to address a 100-year-old conundrum, it could also tell us how vaterite forms in the process of biomineralisation,” says Pokroy. “And if we understand that then it might give us some insight into how to stabilise other metastable forms of calcium carbonate to make them useful.”
Matthew Chalmers
This article originally appeared in the July 2013 edition of ESRFNews.
Top image: Vaterite is the rarest and least stable form of anhydrous calcium carbonate.



