- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- X-ray nanoprobe
- Melanosomes in pigmented epithelia maintain eye lens transparency during zebrafish embryonic development
Melanosomes in pigmented epithelia maintain eye lens transparency during zebrafish embryonic development
Altered levels of trace elements are associated with increased oxidative stress eventually responsible for pathologic conditions, including cataracts. The distribution of elements in the eye of zebrafish embryos were visualised by micro X-ray fluorescence imaging. Eye melanosomes were found to protect the lens from oxidative stress by buffering trace elements.
Trace elements are essential for normal development and physiology of the organism. These elements are found at the core of functional domains of enzymes in nearly all biological pathways. The presence of metal ion transporters with diverse metal affinities and their specific subcellular localisation indicate that each metal needs to be actively transported into proper intracellular compartments. Indeed, mutations in these transporters lead to various medical conditions, including cataracts. To directly visualise sub-cellular element distributions in biological tissues, hard X-ray fluorescence microscopy (μ-XRF) provides unique information. The subcellular distribution of many different elements can be traced quantitatively with a 100-300 nm resolution and over a dynamic range of more than 10,000 fold. At beamline ID22NI (now ID16A), we studied zebrafish as a model organism due to its distinct advantages over other species. It develops rapidly, requiring only 24 hours after fertilisation to form the brain, the heart and the eye. Its small size allowed us to scan the whole eye to map trace element distribution. It also enabled us to examine the consequences of genetic mutations or gene knockdowns.
 |
|
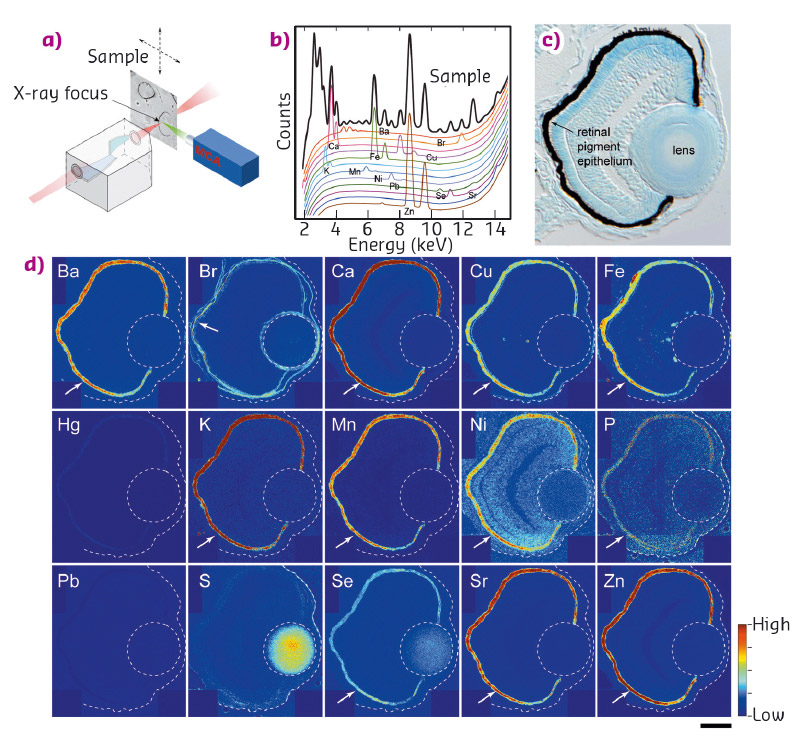
Fig. 42: a) Illustration of µ-XRF imaging setup. b) An example of a fluorescence spectrum. Elements emit X-rays at energies that are characteristic of the given element. c) Transverse 10 µm thick section of 3-day stage zebrafish eye. d) Localisation of elements in the eye. Arrows indicate pigment epithelium. Scale bar in (d): 30 µm. |
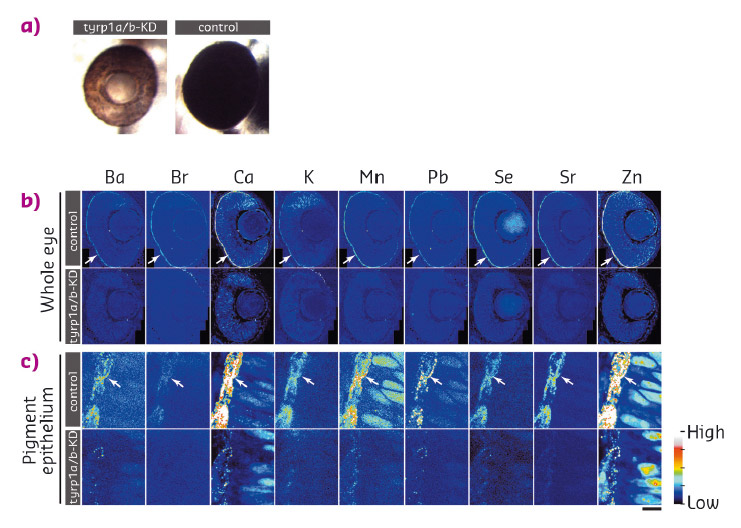
We examined the distribution of 15 elements in the eye. Many elements showed highest accumulation in the retinal pigment epithelium of the zebrafish embryo (Figure 42). To examine whether melanosomes are responsible for the observed enrichment of elements in the pigment epithelial layer, we analysed the distribution of these elements after knockdown of pigment genes (tyrp1a/b) that causes premature brown pigment formation. Knockdown of these zebrafish genes eliminated accumulation of the 15 elements in the pigment epithelium, indicating that they are bound by mature black melanosomes (Figure 43). Furthermore, albino (slc45a2) zebrafish mutants, which completely lack pigment melanosomes, developed abnormal lens reflections similar to the congenital cataract lens. An independent assay to estimate the level of oxidative stress in the tissue revealed increased oxidative stress in the lens of albino mutants. Based on these observations, we hypothesised that defective metal accumulation in the pigment epithelium may increase the risk of cataract formation. To test this idea, we transplanted wildtype lenses into albino mutants with no pigments. We confirmed the cataract formation of the wildtype lens inside pigment-free albino embryos. In conclusion, the results suggest that melanosomes in pigment epithelial cells protect the lens from oxidative stress during embryonic development, likely by buffering trace elements.
 |
|
Fig. 43: a) Knockdown of tyrp1a/b results in the absence of mature melanosomes that produce the brown colour of the pigment epithelium, while control embryos form mature black pigment epithelium. b) Distribution of inorganic elements in the eye of embryos of control (control, upper row) or tyrp1a/b-knockdown group (tyrp1a/b-KD, lower row). c) Higher resolution images were scanned with a 100 nm step size. |
Principal publication and authors
Melanosomes in pigmented epithelia maintain eye lens transparency during zebrafish embryonic development, M. Takamiya (a), F. Xu (b), H. Suhonen (c,e), V. Gourain (a), L. Yang (a), N. Yu Ho (a), L. Helfen (b,c) A. Schröck (a), C. Etard (a), C. Grabher (a), S. Rastegar (a), G. Schlunck (d), T. Reinhard (d), T. Baumbach (b) and U. Strähle (a), Scientific Reports 6, 25046 (2016); doi: 10.1038/srep25046.
(a) Institute of Toxicology and Genetics, Karlsruhe Institute of Technology (KIT) (Germany)
(b) Institute for Photon Science and Synchrotron Radiation (IPS), Karlsruhe Institute of Technology (KIT) (Germany)
(c) ESRF
(d) Eye Center, Freiburg University Medical Center (Germany)
(e) Current address: University of Helsinki, Department of Physics (Finland)



