- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Structure of materials
- The electronic and structural properties of highly luminescent oligoatomic silver clusters confined in zeolites
The electronic and structural properties of highly luminescent oligoatomic silver clusters confined in zeolites
Luminescent silver-zeolite composites with external quantum efficiencies close to 100% were synthesised by applying rational design rules for assembling metal clusters in zeolites. Extended X-ray absorption fine structure combined with photoelectron spectroscopies were utilised to unravel the electronic and structural properties of this novel type of functional material.
Silver clusters consisting of just a few atoms display remarkable optical properties, originating from discrete energy levels in their energy diagram [1]. However, current applications for such oligoatomic clusters are limited due to their tendency to aggregate into larger nanoparticle ensembles, resulting in the loss of their peculiar properties. A strategy to stabilise such clusters is to confine them within the porous framework of zeolites, resulting in stable silver clusters that retain their unique optical properties [2]. Zeolites are minerals that possess a high rigidity and well-defined crystal structure of small molecular-sized cavities that are either found in nature or produced synthetically on an industrial scale.
In this report, the self-assembly of silver clusters in four different types of zeolites, two Linde type A (LTA) and two faujasite type (FAU) zeolites, was studied. These zeolites are all composed of sodalite cages, but differ in their secondary building units and silicon-to-aluminum (Si/Al) ratios, conferring on them different electronic features (Si/Al ratios for LTA, FAUX and FAUY are 1, 1.1, and 2.7, respectively). An in-depth characterisation of these luminescent silver-exchanged zeolites with various advanced spectroscopy techniques provided unambiguous evidence of a strong influence of the zeolite host and degree of silver uptake on the structural, electronic and optical properties of the silver clusters. The deep understanding acquired was crucial to develop rational design rules for assembling metal clusters within zeolites, leading to the synthesis of materials with luminescence efficiencies close to 100% for Ag-FAU zeolites.
 |
|
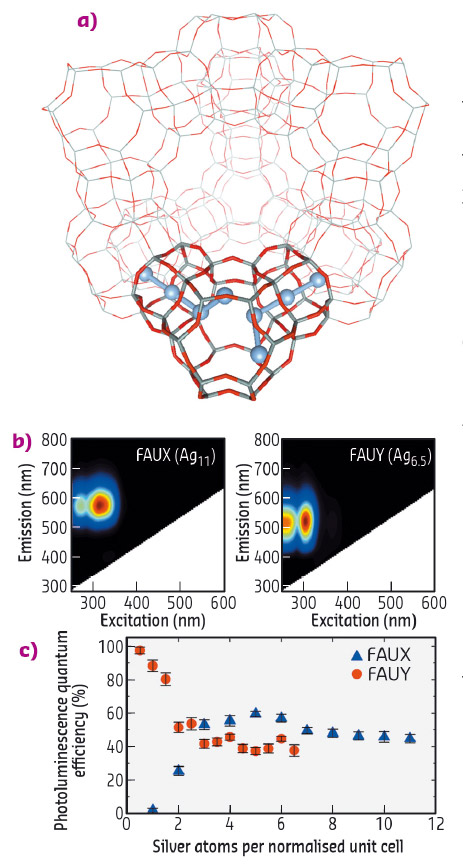
Fig. 57: a) FAU zeolite unit cell displaying a schematic representation of the Ag clusters in a sub-unit cell. b) 2D emission/excitation plot of fully Ag-exchanged FAUX and FAUY zeolites. c) PLQE values of FAUX and FAUY zeolites with different Ag exchange ratios. |
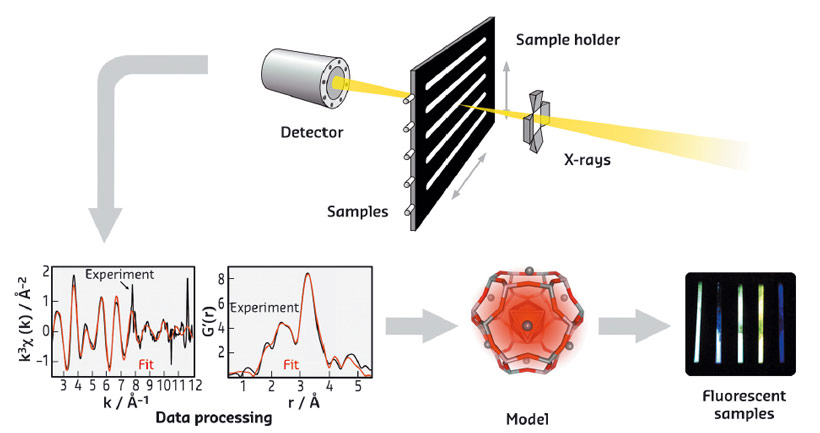
The structural characterisation of these Ag-zeolites was performed by extended X-ray fine structure (EXAFS) analysis at beamlines BM26 (DUBBLE) and BM08 (LISA). In Ag-FAU zeolites, we found a cluster of around 4 atoms, indicative of an Ag4 species (Figure 57a), whereas for clusters stabilised in LTA zeolites, nuclearities between 3 and 6 were observed, suggesting the formation of Ag3 and Ag6 species. Remarkably, significant differences were observed between the Ag-FAU samples possessing different Si/Al ratios (FAUX and FAUY). From the EXAFS model (Figure 58), it was observed that for Ag-FAUX the fraction of silver atoms forming the cluster, changed from 57% to 73% with respect to the silver loading. This observation is in line with the trend found in the photoluminescence quantum yield (PLQY) measurements (Figure 57c), where the PLQY of Ag-FAUX rises (reaching a plateau at around 60%) with increasing silver loading.
 |
|
Fig. 58: Schematic representation of the EXAFS approach followed to characterise luminescent silver clusters confined in zeolites, leading to the optimisation of their optical properties. |
In contrast, Ag-FAUY samples displayed the highest PLQY, amounting to 97% for the lowest Ag loaded sample. This value decreased in samples containing higher silver loadings until reaching a similar plateau as that found in Ag-FAUX samples. However, no significant differences were detected by EXAFS for the fraction of silver atoms forming the clusters in the low-loaded Ag-FAUY sample compared to the other loadings. Detailed analysis of the local order extracted from the Debye-Waller factors showed that the cluster structure in the most efficient low loaded Ag-FAUY sample was significantly more ordered. This counter-intuitive trend in the cluster structure and PLQY values could be explained by a difference in the relative mobility of the Ag+ and Na+ ions inside the two faujasite structures, Ag+ ions possess a larger mobility than Na+ ions in Ag-FAUY zeolites, whereas the opposite is true in Ag-FAUX samples. These findings are of paramount importance to the elucidation of the structure-to-property relationships of small metal clusters and ultimately the design principles established will be crucial for developing highly luminescent Ag-zeolite materials with potential applications in optoelectronics (luminescent tags, fluorescent lamps, LEDs).
Principal publication and authors
Tuning the energetics and tailoring the optical properties of silver clusters confined in zeolites, O. Fenwick (a,b), E. Coutiño-Gonzalez (c), D. Grandjean (d), W. Baekelant (c), F. Richard (a), S. Bonacchi (a), D. De Vos (e), P. Lievens (d), M. Roeffaers (e), J. Hofkens (c) and P. Samori (a), Nat. Mater. 15, 1017-1022 (2016); doi: 10.1038/NMAT4652.
(a) ISIS & icFRC, Université de Strasbourg & CNRS (France)
(b) Present address: School of Engineering and Materials Science, Queen Mary University of London (UK)
(c) Chemistry Department, KU Leuven (Belgium)
(d) Laboratory of Solid State Physics and Magnetism, KU Leuven (Belgium)
(e) Department of Microbial and Molecular Systems, KU Leuven (Belgium)
References
[1] I. Diez and R.H.A. Ras, Nanoscale 3, 1963 (2011).
[2] G. De Cremer et al., J. Am Chem. Soc. 131, 3049 (2009).



