- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Electronic structure and magnetism
- Characterising the elusive axial hydration shell of the Pd(II) aqua ion
Characterising the elusive axial hydration shell of the Pd(II) aqua ion
Chemical behaviour and characterisation of Pd(II) and Pt(II) complexes in solution is a very active area of study because of the large number of their applications in fields such as catalysis, intermediate reaction synthesis and antitumoural treatments. The structure of these metal complexes in solution is characterised by a well defined square-planar arrangement that contrasts with a rather diffuse axial environment. A theoretical proposal for a characteristic hydration shell in this axial region, called the meso-shell [1], stimulated further experimental and theoretical studies which have led to various different pictures.
The present report addresses the question of the experimental detection of this structure by undertaking a recently proposed multitechnique approach which is particularly appropriate for this demanding problem [2]. A set of neutron and X-ray diffraction measurements have been performed; they were combined with a modified reverse Monte Carlo method, empirical potential structure refinement (EPSR) [3], which applies constraints to the local order around the metal cation by means of information derived from the corresponding EXAFS spectrum [4]. These techniques are able to provide structural information on different regions around the central cation with different weightings.
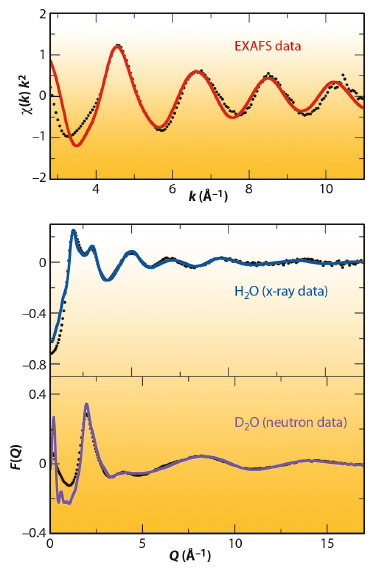
Figure 102 shows the neutron (bottom) and X-ray data (middle) together with the fitted data obtained by the EPSR model. It must be noted that a common atomistic representation provided by the Monte Carlo simulation using the final refined intermolecular potentials is able to simultaneously match the structure of the different diffractograms. The top of Figure 102 shows the comparison of the experimental and simulated k2-weighted Pd K-edge EXAFS spectrum of the Pd(II) aqueous solution obtained at beamline BM29. The simulated EXAFS spectrum was obtained by averaging the individual spectra that result from selecting 10000 local structures from the same MC simulation.
 |
|
Fig. 102: Experimental (black points), fitted neutron diffraction (magenta line), X-ray diffraction (blue line) and simulated EXAFS (red line) spectrum of various Pd(II) aqueous solutions. |
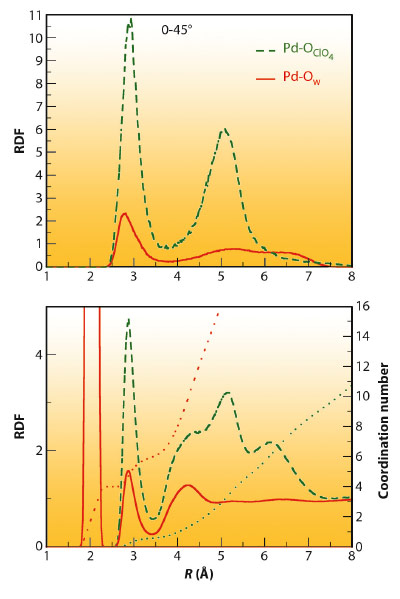
Figure 103 presents the most relevant pair distribution functions (RDF) of the EPSR model that fits the experimental data. The Pd-Ow RDF (bottom) shows a first sharp peak centred at 2.04 Å that integrates to 4 oxygen atoms which belongs to the Pd aqua ion, and a second less intense but well defined peak centred at 2.85 Å that integrates to 2 oxygen atoms. This peak confirms the axial hydration, which encloses a distance range distribution of 2.7-3.5 Å. The second plotted RDF corresponds to the Pd-OClO4 distribution of the perchlorate anions around Pd(II). It is noticeable that the first Pd-OClO4 peak is centred at the same position as the second Pd-Ow peak, and their widths are also similar. Both peaks present the same topology, except for their heights. This is expected due to the preferential attractive character that metal cation aqua ion induces in its closest environment for anions, as well as their relative concentration with respect to water. In fact the running coordination number is 0.5 oxygen atoms for the ClO4- case. This close similarity indicates that the regions above and below the molecular plane of the aqua ion can be envisaged as a hydration shell and also as a coordination region.
 |
|
Fig. 103: Pd-Owater (red line) and Pd-Operchlorate (green line) RDF and running coordination number corresponding to the whole solution (bottom) and the axial region (0-45°) above and below the Pd aquaion molecular plane. |
Due to the molecular plane defined by the Pd(II) aqua ion, a decomposition of the total RDF into partial RDFs could be performed, with each partial RDF corresponding to different regions of the space. The partial Pd-Ow and Pd-OClO4 RDFs corresponding to the axial region (0-45° range of the azimuthal angle) for the Pd-Ow and Pd-OClO4 pairs have been included in Figure 103 (top). These plots support the structural analysis carried out previously. The Pd-Ow peak at 2.85 Å appears in its axial partial RDF, whereas in the axial Pd-OClO4 partial RDF a peak at 2.85 Å is seen for the perchlorate oxygen coordinating the Pd and a second peak at 5 Å belonging to the rest of oxygens of the axial perchlorate anion.
The results confirm the existence of the meso-shell [1] and structurally characterise the water molecules within it. An important finding not previously reported is that the counter ion of the metal cation, the perchlorate anion, competes with water molecules for the meso-shell occupancy. The important role played by the axial region in many ligand substitution reactions is therefore intimately connected to the presence of the counterion and not just hydration water. This highlights the non-secondary role the counterions may play in synthesis.
Principal publication and authors
D.T. Bowron (a), E.C. Beret (b), E. Martín-Zamora (c), A.K. Soper (a) and E. Sánchez Marcos (b), J. Am. Chem. Soc. 134, 962 (2012).
(a) ISIS Facility, Rutherford Appleton Laboratory, Harwell Science and Innovation Campus, Didcot (UK)
(b) Departamento de Química Física. Universidad de Sevilla (Spain)
(c) Departamento de Química Orgánica. Universidad de Sevilla (Spain)
References
[1] J. M. Martínez, F. Torrico, R.R. Pappalardo and E. Sánchez Marcos, J. Phys. Chem. B Soc. 108, 15851 (2004).
[2] D.T. Bowron and S. Díaz-Moreno, J. Phys. Chem.B Soc. 113, 11858 (2009).
[3] A.K. Soper, Phys. Rev. B 72, 104204 (2005).
[4] D.T. Bowron, Pure Appl. Chem. 80, 1211 (2008).



