- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2003
- Industrial and Applied Research
- Template Burning inside Zeolite Framework Monitored by in situ X-ray Powder Diffraction
Template Burning inside Zeolite Framework Monitored by in situ X-ray Powder Diffraction
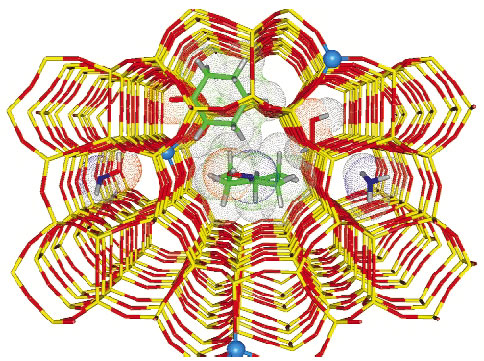
Zeolites are microporous crystalline materials constituted by corner-sharing [TO4] tetrahedra where T = Si or Al (other metals such as Ti, Fe, Ga etc can be introduced into the framework together with or instead of Al). Zeolites are widely employed in the modern industrial chemistry as highly-selective catalysts, because of their molecular-shape selectivity in chemical reactions, which is driven by the size and shape of the internal channels and cavities (Figure 150). The internal structure of zeolites gets organised during their synthesis by organic molecules acting as templates. The framework structure develops progressively around the template by T-O-T connection of simple [TO4] units. However, before being used as catalysts, the internal channels and the cavities of the zeolites have to be made free of the template and this is commonly achieved by heating the zeolite in O2 flux. Insertion of Ti or Fe in the MFI zeolitic lattice results in TS-1 and Fe-MFI materials, which are important catalysts for a number of low-temperature oxidation reactions with aqueous H2O2 as oxidant [1] and in the one-step oxidation of benzene to phenol using N2O as oxidant [2].
 |
|
Fig. 150: Molecular graphics representation of the TS-1 framework after template removal: red sticks O atoms, yellow sticks Si atoms; blue balls Ti atoms (substituting Si). The key reactants and products of the cyclohexanone ammoximation reaction (catalysed inside TS-1), see Figure 151. |
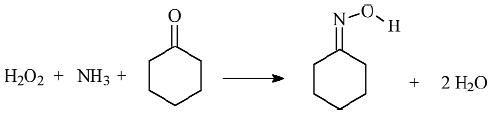
As an example of catalytic reaction inside TS-1, Figure 150 reports a pictorial representation the cyclohexanone ammoximation reaction (a key step in the Nylon 6 production, where cyclohexanone is converted into cyclohexanonoxime). The scheme for this reaction is presented in Figure 151.
 |
|
Fig. 151: The cyclohexanone ammoximation reaction. |
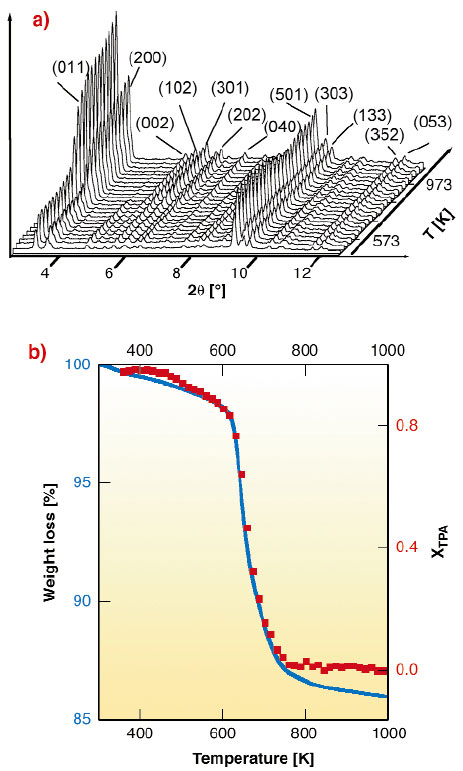
The high X-ray flux available at the ESRF, combined with the use of a suitably-designed area detector setup available on the BM8 beamline (GILDA), allowed to follow the structural changes occurring during the template burning processes inside TS-1 and Fe-MFI zeolites in real time by XRPD (see Figure 152a). The Rietveld full profile analysis yields the template occupancy factor vs. T (Figure 152b, solid squares, right axis) in good agreement with the thermo-gravimetric data. The model for the refinement of the template molecule derives from the single crystal XRD study performed on the ID11 beamline [3].
 |
|
Fig. 152: (a) Evolution of the XRPD patterns in the 2.5-12.5° 2 |
By monitoring the evolution of the a, b and c lattice parameters vs. temperature it has been observed that the volume changes anisotropically during the whole process, for both TS-1 and Fe-MFI, following the inequalities b(T)/b0 < c(T)/c0 << a(T)/a0, being a0, b0 and c0 the starting lattice parameters. These results imply that the zeolite crystals are subject to remarkable stress forces during the burning process, and that the strong anisotropic contraction contributes to crack formation in the zeolite crystals. These data allow us to have, for the first time, a complete view of the structural rearrangements induced by the template burning process on the zeolitic framework.
Isothermal runs have also been performed, and the results of the kinetic analysis indicate that the template burning is a diffusion-limited process with monodimensional advancement. The rate limiting step of the reaction is the diffusion of the volatile products of the burning process out of the crystal. Chemical and steric considerations indicate that the straight channels along the b axis are the more favorable direction for the molecule diffusion and removal. Corresponding Arrhenius plots result in an apparent activation energy values of 151 ± 11 kJmol-1 and of 159 ± 7 kJmol-1 for TS-1 and Fe-MFI, respectively.
References
[1] S. Bordiga, A. Damin, F. Bonino, G. Ricchiardi, C. Lamberti and A. Zecchina,. Angew. Chem. Int. Edit. 41, 4734-4737 (2002).
[2] G. Berlier, G. Spoto, S. Bordiga, G. Ricchiardi, P. Fisicaro, A. Zecchina, I. Rossetti, E. Selli, L. Forni, E. Giamello and C. Lamberti, J. Catal., 208, 64-82 (2002).
[3] L. Palin, C. Lamberti, Å. Kvick, F. Testa, R. Aiello, M. Milanesio and D. Viterbo, J. Phys. Chem. B, 107, 4034-4042 (2003).
Principal publication and Authors
M. Milanesio (a), G. Artioli (b), A.F. Gualtieri (c), L. Palin (d,e), C. Lamberti (d), J. Am. Chem. Soc., 125, 14549-14558 (2003).
(a) University of Piemonte Orientale (Italy)
(b) University of Milano (Italy)
(c) University of Modena (Italy)
(d) University of Torino (Italy)
(e) now at the ESRF (France)



