- Home
- News
- Spotlight on Science
- Meningitis bacteria...
Meningitis bacteria stealth tactics revealed
06-05-2009
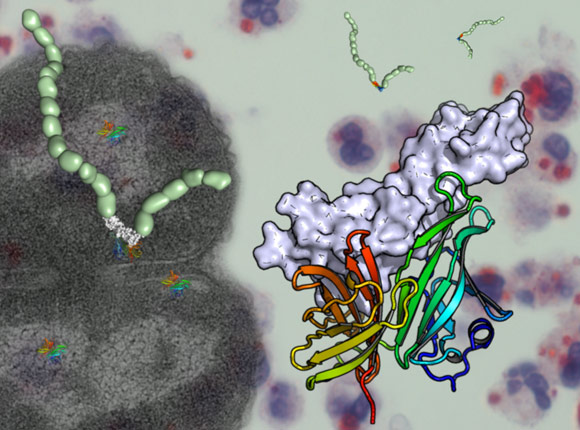
Meningitis bacteria mimic human cells to avoid detection by our immune system. The crystal structure of a Neisseria meningitidis surface protein in complex with human factor H shows us how this happens and may pave the way for a new vaccine against the disease.
Meningitis involves an inflammation of the membranes surrounding the brain and the spinal cord as the result of an infection. The infection can be due to viruses or bacteria, but bacterial meningitis is much more serious, with approximately 5% of cases resulting in death. Although the disease mainly affects infants and young children, it is also often found in teenagers and young adults. The disease is frightening because it can strike rapidly, with people becoming seriously ill within hours. The bacterium Neisseria meningitidis is the most common cause of bacterial meningitis. It comes in different forms, causing different strains of the disease. With vaccines against strains A and C, group B now accounts for around 90% of cases in the UK. While there is still no vaccine available for strain B, two vaccine candidates are in clinical trials.
The complement system, part of the human immune system, is designed to attack all foreign bodies that come into contact with the blood including bacteria. Particular sugar molecules on the surface of human cells flag them as being native to the body and stop them from being attacked and killed. Factor H, a molecule that circulates in the blood, binds to these sugars preventing an autoimmune response. To prevent themselves being destroyed by the immune system, bacteria have a protein, factor H binding protein, that makes their cells appear human in nature.
The crystal structure of a complex between a portion of human factor H and a surface protein that also binds factor H from Neisseria meningitidis has recently been revealed. The structure was solved using diffraction data collected at the ESRF (ID14-4, ID29, BM14) and at DIAMOND (UK). The data from ID29 were collected via remote access to the beamline. The structure of the complex shows that the surface protein of Neisseria meningitidis mimics precisely the sugars on the surface of human cells thus enabling the bacteria to bind factor H in the same way as human cells.
The crystal structure also identified two amino acids (Glu 304, Glu 341) in the bacterial protein that are crucial to allow its binding to factor H. When these two amino acids were mutated, surface proteins no longer bind factor H. The information provided by the crystal structure could thus lead to better protection against meningitis B, as vaccines containing the mutated bacterial protein may provoke a better immune response than those currently available.
Principal publication and authors
M.C. Schneider (a), B.E. Prosser (a), J.J.E. Caesar (b), E. Kugelberg (a), S. Li (a), Q. Zhang (a), S. Quoraishi (b), J.E. Lovett (b), J.E. Deane (b), R.B. Sim (c), P. Roversi (b), S. Johnson (b), C.M. Tang (a), S.M. Lea (b), Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates, Nature 458, 890-893 (2009).
(a) Centre for Molecular Microbiology and Infection, Imperial College, London (UK)
(b) Sir William Dunn School of Pathology, University of Oxford (UK)
(c) MRC Immunochemistry Unit, Department of Biochemistry, University of Oxford (UK)