- Home
- News
- General News
- Deciphering the...
Deciphering the mechanism of an anti-obesity hormone
24-03-2017
Obesity-related illness, such as type 2 diabetes or cardiovascular diseases are linked to the lack of a hormone called adiponectin. Researchers at the Inserm à l’Institut de Génomique Fonctionnelle, Humboldt Universität in Berlin (Germany), University of Oxford (UK) and the Suez Canal University (Egypt) have unveiled the functioning of this hormone using the ESRF. Their results could open doors to potential treatment for these pathologies.
Adiponectin is a hormone produced by the so-called adipocytes, cells that stock lipids. It controls the general energetic balance in the body by regulating the use of glucose and fat. Levels of adiponectin are higher in lean people than in obese individuals, and they are correlated with fat-cell health and a lower risk of developing diabetes and heart disease. Unfortunately, until today no one has managed to unveil how the hormone works and how it links to its receptors.
The international team of scientists, led by Sébastien Granier, has focused on the receptors, called ADIPOR1 and ADIPOR 2, which are membrane proteins located at the surface of the cell. The researchers came to the ESRF’s macromolecular crystallography beamlines, where they solved the atomic structure of ADIPOR2 and revised the published data for ADIPOR1. Thanks to enzymology experiments and bioinformatics they fine-tuned the adiponectin’s mechanism of action when it binds to the receptors.
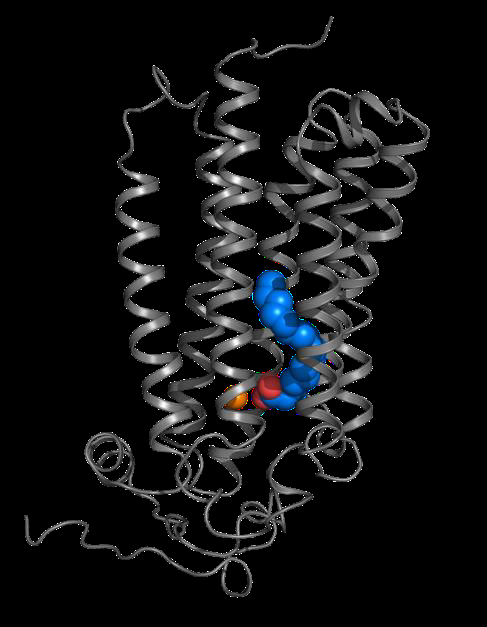 |
|
Visualisation of ADIPOR2 in 3D (grey) interacting with a fatty acid (blue and red) and a zinc atom (orange) involved in the hydrolysis reaction. Credits: S. Granier. |
Mesh and collect
“The mesh and collect method at the ESRF has allowed us to detect fatty acids in pockets of ADIPOR2. We couldn’t have done this without this method, as our crystals were too small”, explains Sébastien Granier, director of research at the Institut de Génomique Fonctionnelle
“Mesh & Collect” is a development carried out by the ESRF with the Synchrotron Serial Crystallography BAG (beamline allocation group), inspired by recent developments made at X-ray Free Electron Lasers. The method consists of scientists harvesting many small crystals directly from crystallisation drops using a mesh sample holder. This sample holder is then scanned in two dimensions at cryogenic temperatures using a low intensity X-ray beam to determine the positions of individual crystals by X-ray diffraction. From those crystals, scientists record partial diffraction data sets over a small rotation range. After sorting the individual partial data sets in clusters of isomorphous similarity, these are merged to give a complete diffraction data set that can be used for structure solution.
The ESRF has integrated this method in the last years and it is popular among users. "This method allows us to do experiments which would not have been possible a couple of years ago. It is available as an automatic workflow, ready for users to exploit it and it can play a big role in research, like in the case of Granier's team study", explains ESRF scientisit Christoph Mueller-Dieckman.
Antidiabetic potential
Diabetes experts William L. Holland and Philip E. Scherer, from the Touchstone Diabetes Center in the University of Texas Southwestern Medical Center, comment on the potential of this work for diabetes treatment in a related article in Nature: “The activation of signalling pathways downstream of adiponectin might help to prevent the development of insulin resistance and type 2 diabetes. The current study should greatly aid the quest to design drugs that promote ADIPOR activity. Adiponectin mimetics might unleash the full antidiabetic potential of these receptors.”
Granier is already thinking of how to make this mimetics possible, and is already keenly eyeing the next development at the ESRF for his future experiments: “The ESRF is developing a CryoEM (electron microscope) which would help us answer many of the questions we still have. It would be fantastic if we could make use of this new microscope in our structural biology research when it comes to life later in the year”.
Publication
Structural insights into adiponectin receptors suggest ceramidase activity - I. Vasiliauskaite-Brooks, R. Sounier, P. Rochaix, G. Bellot, M. Fortier, F. Hoh, L. De Colibus, C. Bechara, E. M. Saied, C. Arenz, C. Leyrat, S. Granier, Nature (2017); doi: 10.1038/nature21714
Text by Montserrat Capellas Espuny
Top image: 3D visualisation of ADIPOR 1 en mode surface (light gray) with a ceramide (yellow and red). Credits: S. Granier.



