- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2016
- Structure of materials
- Na-rich Na4V2(PO4)2F3 for advanced Na-ion batteries
Na-rich Na4V2(PO4)2F3 for advanced Na-ion batteries
A scalable ball milling synthesis involving metallic sodium was used to prepare the Na-rich Na4V2(PO4)2F3 cathode for Na-ion batteries. This method provides additional Na to compensate Na loss in solid electrolyte interface formation and thus brings a substantial increase in the energy density.
Na-ion batteries are the most appealing alternative to current Li-ion batteries due to the natural abundance of sodium which is expected to bring a 20% cost decrease [1]. Of the candidate materials for the cathode, the polyanionic compound Na3V2(PO4)2F3 is of great interest since it shows high voltage plateaus near 3.6 and 4.2 V whose equal amplitudes provide a cumulative capacity of ~ 120 mAh g-1 [2]. To make practical cells, the Na3V2(PO4)2F3 positive electrode is matched with hard carbon having a reversible capacity of 300 mAh g-1, out of which 25% is lost to form the solid electrolyte interface (SEI) during the first cycle. Owing to the low coulombic efficiency of hard carbon in Na-ion batteries, i.e., less than 80%, a strategy to compensate for Na loss to the SEI is vital.
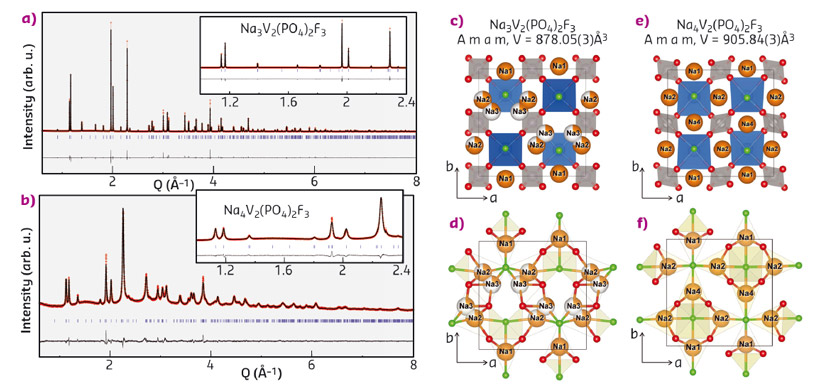
We report herein the synthesis of Na-rich Na4V2(PO4)2F3 compounds via the ball milling of Na-metal with Na3V2(PO4)2F3, where the additional Na is used to build SEI on the anode side in a full cell. Na3V2(PO4)2F3 powders were mixed with a metallic lump of Na and ball milled in a SPEX 8000 milling apparatus. A single phase of Na4V2(PO4)2F3 was obtained after 3 hours of ball milling. X-ray diffraction patterns collected at beamline ID22 of the pristine Na3V2(PO4)2F3 and Na4V2(PO4)2F3 phases are shown in Figure 52a-b, respectively. The diffraction peaks of the phase formed upon ball milling with Na can be indexed in the same orthorhombic cell as for the pristine Na3V2(PO4)2F3, but with different lattice parameters, i.e. a = 9.2208(2) Å, b = 9.2641(2) Å, c = 10.6036(2) Å. This corresponds to an increase of the unit cell by 3.2% (V = 905.79(3) Å3) relative to the pristine Na3V2(PO4)2F3 phase (V = 878.05(3) Å3), which is consistent with the uptake of extra sodium upon reduction. The Na environments in the Na-rich phase are shown in Figure 52e-f. The three distinct Na sites are all 7-fold coordinated with 4 oxygen and 3 fluorine atoms, which is analogous to the coordination of Na1 in Na3V2(PO4)2F3 (Figure 52c-d). The structural analysis confirms the chemical composition (Na4V2(PO4)2F3) and indicates that there is apparently no further space for Na insertion.
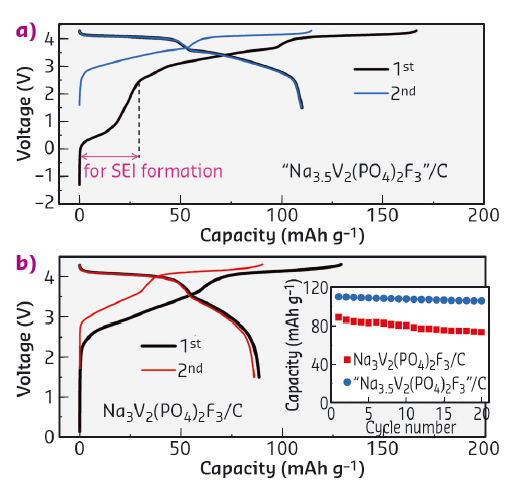
The benefits of preparing Na-rich Na4V2(PO4)2F3 is demonstrated through assembling full cells with hard carbon anodes. Figure 53 shows the electrochemical performances of cells C1 (blue) and C2 (black), having pristine Na3V2(PO4)2F3 and composite "Na3.5V2(PO4)2F3" (i.e., equal amount of Na3V2(PO4)2F3 and Na4V2(PO4)2F3) as positive electrodes, respectively. The cells were tested electrochemically between 1.5 and 4.3 V. The voltage trace for C1 mirrors early literature reports for similar cells with a charging capacity of 129 mAh g-1 and a discharge capacity of 89 mAh g-1, which remains stable upon cycling. Providing sacrificial Na in the form of Na-rich NVPF (C2) strongly modifies the voltage profile. Note the existence of an initial capacity near 0.5 V which corresponds to the removal of Na from "Na3.5V2(PO4)2F3" to compensate for the SEI formation at the negative electrode. Afterwards the potential rises as expected with associated removal of Na from Na3V2(PO4)2F3. Overall, the C2 cell exhibits an overall charging capacity of 167 mAh g-1 and a discharge capacity of 110 mAh g-1, which is a 24% enhancement compared to cell C1. This corresponds to an overall 10% increase in energy density. Lastly, we have shown that the use of the Na-rich phase does not jeopardise the cycle life, since the capacity remains nearly constant over 20 cycles.
 |
|
Fig. 53: Performance of C/"Na3+xV2(PO4)2F3" Na-ion cells. Inset in (b) shows the capacity retention in the first 20 cycles. |
The means to enhance the performance of Na-ion batteries discussed here should have a very positive impact on their future development for commercialisation.
Principal publication and authors
Insertion compounds and composites made by ball milling for advanced sodium-ion batteries, B. Zhang (a,b,c), R. Dugas (a,b,c), G. Rousse (a,b,c,d), P. Rozier (b,c,e), A.M. Abakumov (f,g) and J.-M. Tarascon (a,b,c,d), Nature Communications 7, 10308 (2016); doi: 10.1038/ncomms10308.
(a) Chimie du Solide-Energie, UMR 8260, Collège de France, Paris (France)
(b) Réseau sur le Stockage Electrochimique de l’Energie (RS2E), FR CNRS 3459, Amiens (France)
(c) ALISTORE-European Research Institute, Amiens (France)
(d) Sorbonne Universités - UPMC Univ Paris 06, Paris (France)
(e) University of Toulouse III Paul Sabatier, CIRIMAT CNRS UMR 5085, Toulouse (France)
(f) EMAT, University of Antwerp, Antwerp (Belgium)
(g) Skolkovo Institute of Science and Technology, Moscow (Russia)
References
[1] D. Larcher et al., Nat. Chem. 19, 7, (2014).
[2] M. Bianchini et al., Chem. Mater. 4238, 26, (2014).