- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Complex systems and biomedical sciences
- Differences in gene expression after microbeam radiation therapy
Differences in gene expression after microbeam radiation therapy
The efficiency of microbeam radiation therapy (MRT) to improve the survival of tumour bearing rats is already well documented. A description of early molecular events after MRT could complete our understanding of the biology of this particular irradiation. By the use of microarray gene technology, we have previously documented the whole transcriptomic response of normal and tumour brain tissues to MRT and more recently identified genes or molecular pathways potentially involved in MRT efficiency or tumour resistance to this treatment.
Microbeam radiation therapy is a novel kind of radiation therapy which uses arrays of quasi-parallel low-energy photons. Beamline ID17 provides photons with an energy range of 40 - 350 keV, a very high photon flux and a low divergence that fulfills the requirements of collinearity and dose rate for MRT necessary to produce plane parallel spatially-fractionated microbeams. Preclinical research in small animal models has shown tumour control by MRT, while offering an excellent level of normal tissue sparing.
The preferential controlling effects observed for tumour vessels may not entirely explain the efficiency of MRT and other biological mechanisms may be involved in tumour control. On a well characterised model (9L gliosarcoma) [1], the early whole transcriptomic responses of normal and tumoural tissues 6h after unidirectional MRT (400 Gy, 50 µm-wide microbeams, 200 µm spacing) (Figure 93) and the associated biofunctions and pathways have already been described [2]. This provided a data base with the expression of 28,000 transcripts which has allowed the identification of transcripts involved in both tumour and normal brain responses to MRT [1].
 |
|
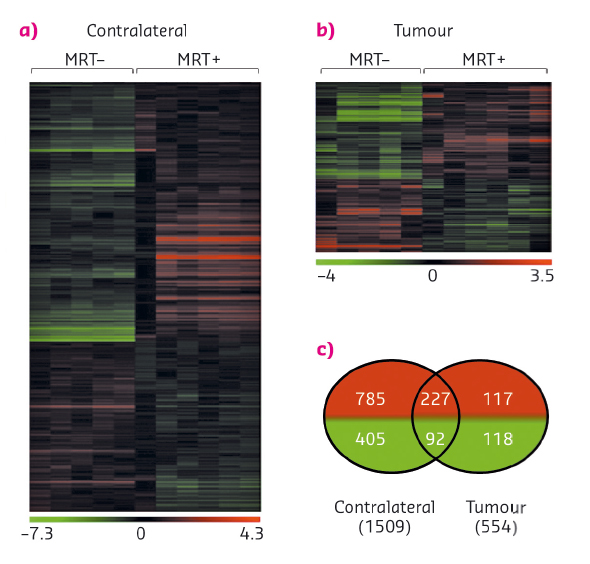
Fig. 93: Influence of MRT on gene expression. a) and b) Heat map showing either significant loss or gain of mRNA expression after MRT in tumour and normal brain tissues. Colours indicate expression levels above (red) or below (green) the median for each gene. Vertical columns indicate individual arrays and horizontal rows indicate genes. c) Venn diagrams showing the numbers of significantly increased (red) or decreased (green) genes after MRT in tumour and contralateral brain tissues [2]. |
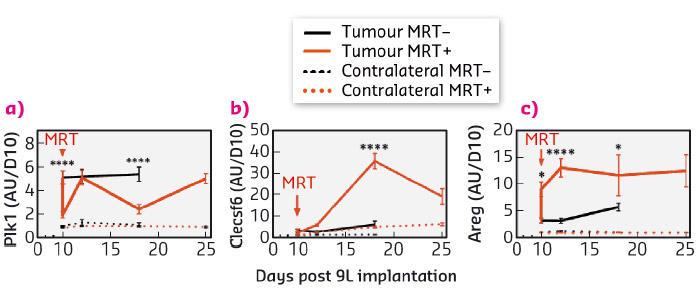
The specific response of tumour tissues to MRT consisted of the significant transcriptomic modulation of 316 transcripts (431 probsets) which are mainly related to the regulation of cell cycle and the immune/inflammatory response. Of these, 30 were not detected in normal brain tissues, neither before nor after MRT and could be divided into 3 groups of relevant targets. These targets were monitored over several days and weeks after irradiation and their modulation was studied. In summary (i) Polo-like kinase 1 (Plk1), known to be necessary for efficient cell division and proposed as potential therapeutic targets in human tumours, showed decreased expression a fews hours and a fews days after MRT (Figure 94a). A cluster of 20 genes (including Ccnb1, Cdc20 and Pttg1) presented the same kinetic of expression and may partially explain the control of tumour growth by MRT through the deregulation of cell division - they could be amplified to continue the tumour growth inhibition; (ii) in tumours, the expression of the 7 transcripts of Clec group, related to immune cells, was inhibited 6 h after MRT but increased significantly 2, 8 and 15 days after MRT (Figure 94b). Clecsf6 is known to be expressed at the surface of monocytes, macrophages and dendritic. The kinetic of transcriptomic expression of this group coincides temporally with the presence of leukocytes in tumour tissues, a fact that may indicate a progressive recruitment of immune cells in the tumour after MRT. (iii) Areg (encoding for amphiregulin) is overexpressed in tumours after MRT (Figure 94c) and its involvement described in the literature for chimio/radioresistance suggests that its inhibition might help in tumour control after MRT.
 |
|
Fig. 94: Kinetic expression of genes from three target groups associated with brain tumour tissue response to MRY. Transcriptomic expression of Plk1 (a), Clecsf (b) and Areg (c) measured 6 hours, 2, 4, 8 and 15 days after MRT. Untreated are presented in black, MRT-treated in red; normal and tumour tissue in dashed and solid lines respectively. |
Principal publication and authors
Identification of AREG and PLK1 pathway modulation as a potential key of the response of intracranial 9L tumour to microbeam radiation therapy, A. Bouchet (a,b), N. Sakakini (c,d), M. El Atifi (a,e), C. Le Clec’h (b), E. Bräuer-Krisch (b), L. Rogalev (b), J. Laissue (f), P. Rihet (c,d), G. Le Duc (b) and L. Pelletier (a,e), Int J Cancer 136, 2705-2716 (2015); doi: 10.1002/ijc.29318.
(a) INSERM U836, Team Nanomedicine and brain, La Tronche (France)
(b) ESRF
(c) UMR1090 TAGC, INSERM, Marseille (France)
(d) Aix-Marseille Université, Marseille (France)
(e) Grenoble University Hospital, Grenoble (France)
(f) University of Bern, Bern (Switzerland)
References
[1] A. Bouchet et al. Tumour Biol 35, 6221–6233 (2014).
[2] A. Bouchet et al. PLoS One 8, e81874 (2013).



