- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Soft condensed matter
- Shaping vesicles by the presence of amphiphilic copolymer
Shaping vesicles by the presence of amphiphilic copolymer
Vesicles are globular objects of closed amphiphilic bilayers, which are formed spontaneously by phospholipids but also by a large number of surfactants and amphiphilic polymers. With their bilayer structure, unilamellar vesicles resemble natural membranes and they are often used in a number of applications including cosmetics, pharmaceutical formulations and detergents. However, for many reasons, it is very important to have precise control over the vesicle size and their stability, as frequently vesicles are metastable and structurally not so well-defined.
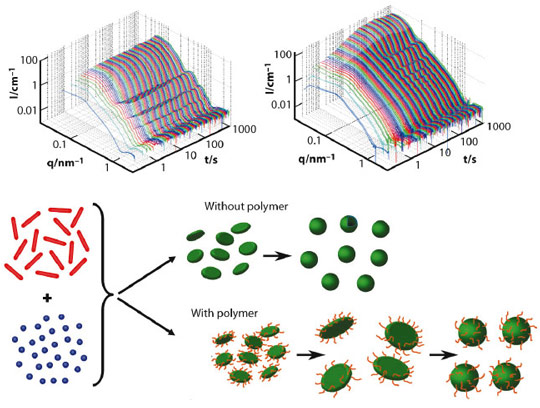
In the past, we have studied the spontaneous formation of vesicles occurring upon rapidly mixing either oppositely charged surfactants or mixtures of anionic and zwitterionic surfactants. Very detailed structural information regarding the formation process could be obtained by stopped-flow SAXS experiments down to a time resolution of 2 ms. These experiments showed that very small disk-like micelles form initially, grow by coalescence and then, once beyond a critical size, close spontaneously to form monodisperse vesicles [1]. This critical size is basically determined by an energetic balance between the unfavourable rim of the disk and the energy required to bend the bilayer into the curved vesicles [2,3].
A logical idea then is to make this rim more stable, thereby forming correspondingly larger vesicles. This concept has been realised by adding an amphiphilic copolymer, in our case of the PEO-PPO-PEO type (EO: ethylene oxide, PO: propylene oxide), which can be expected to become enriched in the rim of the disk-like micelles and thereby reduce the line tension. Here we have studied the corresponding process of vesicle formation in the presence of the amphiphilic copolymer in order to verify this hypothesis. We obtained detailed structural information about the progression of vesicle formation by time-resolved SAXS performed at beamline ID02. Examples for such sets of SAXS curves, with and without added copolymer, are shown in Figure 75 and demonstrate clearly the growth of the small disks, which formed immediately (t < 5 ms), and the subsequent closure to form vesicles (marked in the SAXS curves by the occurrence of oscillations in the form factor). The progression is basically similar to the case without added copolymer but the growth proceeds for much longer, this prolonged growth being proportional to the amount of added copolymer. This then leads to larger vesicles (as seen by the much higher scattering intensity), which are highly monodisperse (as seen from the pronounced form factor oscillations).
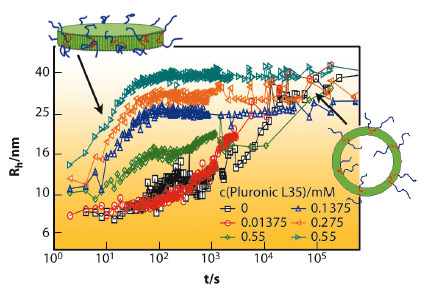
This kinetic control of the vesicle formation process yields very well-defined vesicles of controlled size and, in addition, these copolymer modified vesicles are much more stable than without copolymer. This is demonstrated in Figure 76 which shows dynamic light scattering (DLS) results as a function of time. The presence of the copolymer largely suppresses ageing and systems that were stable before for only a few minutes are now stable for weeks due to the copolymer addition, a point very important for applications.
 |
|
Fig. 76: Hydrodynamic radius Rh from DLS as a function of time after mixing together 27.5 mM TDMAO and 22.5 mM LiPFO in the presence of different concentrations of Pluronic L35 (at 25°C). |
In summary, it can be stated that, for this model of mixed surfactants, a new way of a rational structural design of self-assembled systems has been employed, based on detailed knowledge of the formation process, which is uniquely accessible by highly time resolved SAXS experiments. In our experiments the self-assembly process was manipulated in a systematic way, in a fashion similar to modifying a chemical reaction by a catalyst, i.e. one could call this “colloid catalysis”, which tailors the vesicle shape in our example. In this way, self-assembly of metastable systems leads to well-defined systems, otherwise inaccessible, and with much enhanced stability. This opens access to novel systems, which should also be of great importance for future developments in formulating self-assembled systems.
Principal publication and authors
K. Bressel (a), M. Muthig (a), S. Prévost (a,b), J. Gummel (c), T. Narayanan (c) and M. Gradzielski (a), ACS Nano 6, 5858-5865 (2012).
(a) Stranski Laboratorium für Physikalische und Theoretische Chemie, Institut für Chemie, Technische Universität Berlin (Germany)
(b) Helmholtz-Zentrum Berlin (HZB) für Materialien und Energie GmbH (Germany)
(c) ESRF
References
[1] T.M. Weiss, T. Narayanan and M. Gradzielski, Langmuir 24, 3759-3766 (2008).
[2] W. Helfrich, Phys. Lett. 50A, 115–116 (1974).
[3] A. Shioi, T.A. Hatton, Langmuir 18, 7341-7348 (2002).